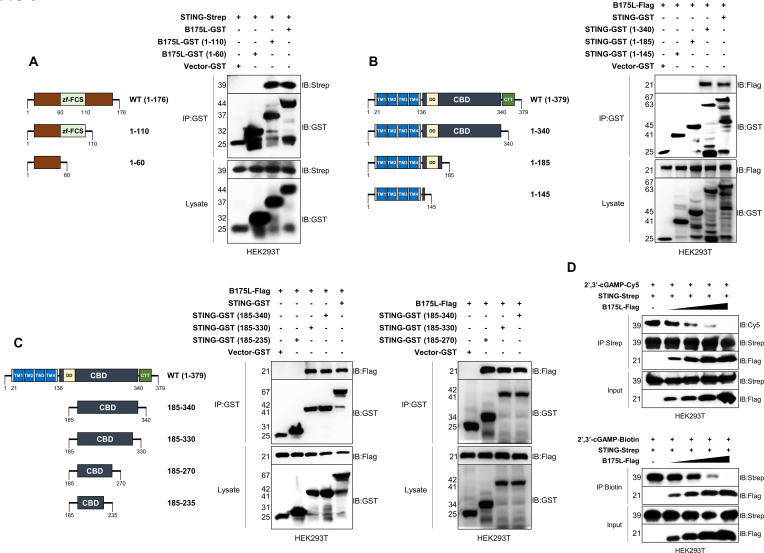
Fig 5.
B175L inhibits STING and cGAMP interaction. (A) GST-tagged B175L domains (aa 1–60 and aa 1–110) with or without the zf-FCS motif (left). Immunoprecipitation of GST-tagged B175L and its domain constructs with STING-Strep (right). Protein expressions were determined by anti-GST and anti-Strep antibodies. (B and C) Domain analysis to find the interface of STING that interacts with the zf-FCS motif of B175L-Flag. Control plasmid, GST-tagged STING (wild type), and its constructs (aa 1–145, aa 1–185, aa 1–340, aa 185–235, aa 185–270, aa 185–330, and aa 185–340) were transfected into HEK293T cells and subjected to GST immunoprecipitation followed by immunoblotting with anti-Flag and anti-GST antibodies. (D) In vitro competition assay with cGAMP, STING, and B175L. 2′3′-cGAMP-Cy5 conjugate or 2′3′-cGAMP-Biotin conjugate was incubated with affinity-purified STING and B175L (1, 3, 6, and 10 µg). After the incubation, the reaction mixture was pulled down with Strep beads or Dynabeads M-280 Streptavidin and subjected to immunoblotting analysis. Protein sizes are expressed in kilodaltons (kDa). All the immunoblot data are representative of at least two independent experiments, each with similar results.