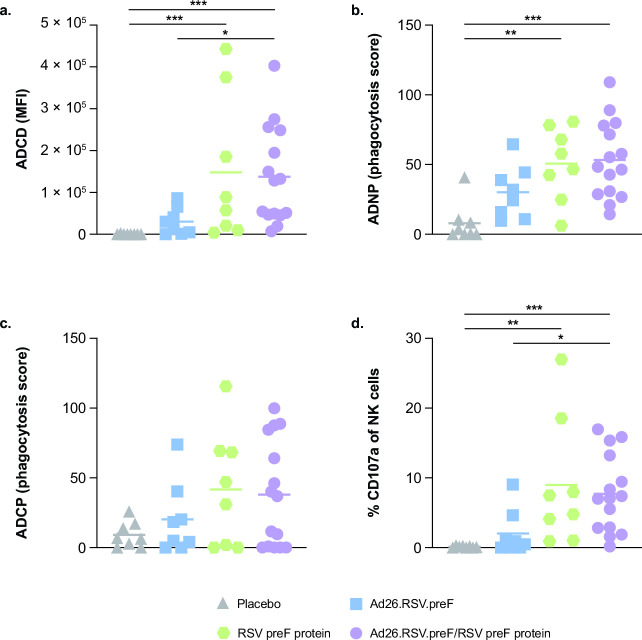
Fig 5.
RSV preF-specific Fc-effector functions induced by Ad26.RSV.preF, RSV preF protein, and the Ad26.RSV.preF/RSV preF protein combination vaccine. RSV preF-specific (a) ADCD, (b) ADNP, (c) ADCP, and (d) ADNKA were measured in serum samples collected at day 15 for participants receiving the Ad26.RSV.preF/RSV preF protein combination vaccine (n = 15), Ad26.RSV.preF alone (n = 8), RSV preF protein alone (n = 8), or placebo (n = 8). Responses at day 15 were baseline corrected (day 0, prior to vaccination), and only positive values are shown. Horizontal lines denote geometric mean values in each group. Statistical comparisons were performed by non-parametric analysis of variance (Kruskal-Wallis) with Benjamini-Hochberg correction for multiple comparisons. *P ≤ 0.05; **P ≤ 0.01; ***P < 0.001. Ad26, adenovector type 26; ADCD, antibody-dependent complement deposition; ADCP, antibody-dependent cellular phagocytosis; ADNKA, antibody-dependent natural killer cell activation; ADNP, antibody-dependent neutrophil phagocytosis; MFI, median fluorescence intensity; NK, natural killer; preF, pre-fusion conformation-stabilized RSV F protein; RSV, respiratory syncytial virus.