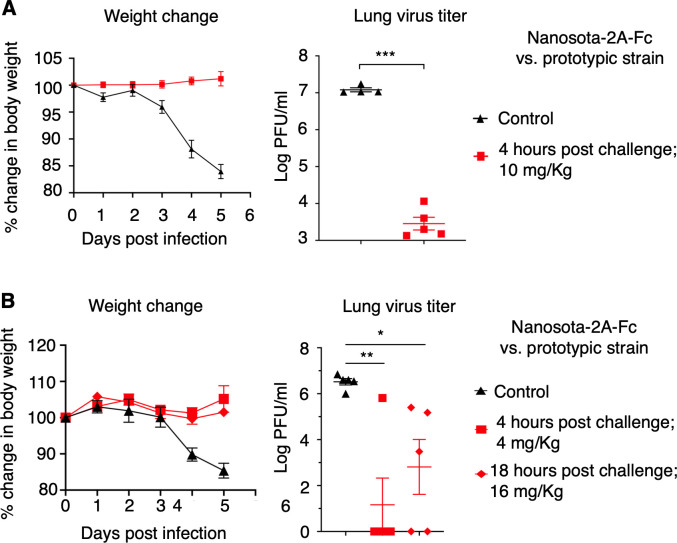
Fig 4.
Nanosota-2 demonstrates super potency against prototypic SARS-CoV-2 in mouse model. The efficacy of Nanosota-2A-Fc was evaluated for treating the infection of prototypic SARS-CoV-2 in mice. (A) Nanosota-2A-Fc was administered at a dosage of 10 mg/kg weight and a time point of 4 h post-challenge. Human ACE2-transgenic mice were challenged via intranasal inoculation of prototypic SARS-CoV-2. In the treatment group (n = 5), mice received Nanosota-2A-Fc via intraperitoneal delivery. In the control group (n = 4), mice were administered PBS buffer. Body weight changes up to day 5 post-challenge were recorded and lung virus titers on day 2 post-challenge were measured. (B) Nanosota-2A-Fc was administered at a reduced dosage (4 mg/kg weight at 4 h post-challenge) or a delayed time point (18 h post-challenge at 16 mg/kg weight). n = 5 for both the treatment and control groups. Comparisons of lung virus titers between the control and treatment groups were performed using unpaired two-tailed Student’s t-test. Error bars represent SEM. *P < 0.05; **P < 0.01; ***P < 0.001.