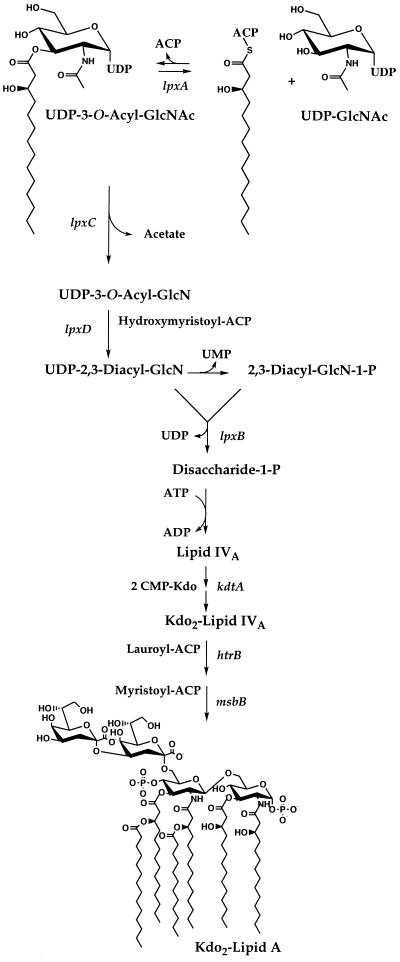
FIG. 1.
Role of LpxA in lipid A biosynthesis in E. coli. LpxA catalyzes the first step of lipid A biosynthesis (24, 25). The transfer of an R-3-hydroxyacyl group from R-3-hydroxyacyl–ACP to UDP-GlcNAc is very selective for R-3-hydroxymyristoyl–ACP in the case of E. coli LpxA (3, 33). UDP-GlcNAc acyltransferases from other gram-negative bacteria have specificity for ACP thioesters of different acyl chain lengths (33). Biosynthetic intermediates and known genes encoding the enzymes of the rest of the E. coli pathway are shown (24, 25). Chemical hydrolysis of lipopolysaccharide or of whole cells at pH 4.5 in the presence of sodium dodecyl sulfate cleaves the 3-deoxy-d-manno-octulosonic acid (Kdo)-lipid A linkage without disturbing the phosphates (7, 8). The released lipid product is designated lipid A throughout this paper. The 10-carbon-specific Pseudomonas LpxA can replace the 14-carbon-specific E. coli enzyme in living cells without interfering with the functioning of the other enzymes of the pathway.