ABSTRACT
Background
Plasma (p-)activin A is elevated in chronic kidney disease–mineral and bone disorder (CKD-MBD). Activin A inhibition ameliorates CKD-MBD complications (vascular calcification and bone disease) in rodent CKD models. We examined whether p-activin A was associated with major adverse cardiovascular events (MACE), all-cause mortality and CKD-MBD complications in CKD patients.
Methods
The study included 916 participants (741 patients and 175 controls) from the prospective Copenhagen CKD cohort. Comparisons of p-activin A with estimated glomerular filtration rate (eGFR), coronary and thoracic aorta Agatston scores, and bone mineral density (BMD) were evaluated by univariable linear regression using Spearman's rank correlation, analysis of covariance and ordinal logistic regression with adjustments. Association of p-activin A with rates of MACE and all-cause mortality was evaluated by the Aalen–Johansen or Kaplan–Meier estimator, with subsequent multiple Cox regression analyses.
Results
P-activin A was increased by CKD stage 3 (124–225 pg/mL, P < .001) and correlated inversely with eGFR (r = −0.53, P < 0.01). P-activin A was associated with all-cause mortality [97 events, hazard ratio 1.55 (95% confidence interval 1.04; 2.32), P < 0.05] after adjusting for age, sex, diabetes mellitus (DM) and eGFR. Median follow-up was 4.36 (interquartile range 3.64–4.75) years. The association with MACE was not significant after eGFR adjustment. Agatston scores and BMD were not associated with p-activin A.
Conclusion
P-activin A increased with declining kidney function and was associated with all-cause mortality independently of age, sex, DM and eGFR. No association with MACE, vascular calcification or BMD was demonstrated.
Keywords: activin A, CKD-MBD, MACE, renal osteodystrophy, vascular calcification
KEY LEARNING POINTS.
What was known:
Plasma (p-)activin A is increased in chronic kidney disease (CKD).
In rodent CKD models, inhibiting activin A slows the progression of renal disease and the consequences of mineral and bone disorder.
This study adds:
This study, which included controls and CKD stage 1–5ND (no dialysis) patients, is the largest to show that p-activin A rises with declining kidney function.
P-activin A was independently associated with all-cause mortality.
P-activin A cannot be used as a biomarker of bone mineral density or vascular calcification.
Potential impact:
Activin A is a promising target for therapeutic intervention.
Strategies aiming at decreasing p-activin A might prove beneficial to CKD-related mortality.
Further study regarding the pathophysiological changes related to activin A signalling in CKD is warranted.
INTRODUCTION
Chronic kidney disease–mineral and bone disorder (CKD-MBD) is a syndrome of dysregulated mineral metabolism including derangement of minerals and hormones together with bone abnormalities, vascular calcification and cardiomyopathy, which progresses with declining kidney function [1]. Ultimately, this leads to fractures, cardiovascular events and mortality [1, 2].
There is an urgent need to develop readily accessible and cost-effective methods to determine the risk of CKD-MBD complications. Contemporary methods in clinical practice primarily rely on traditional biochemical plasma parameters like calcium, phosphate, vitamin D and parathyroid hormone (PTH). However, all these parameters are still suboptimal in the assessment of CKD-MBD with respect to vascular calcification [3] and bone disease [4] in patients with CKD. Several studies have demonstrated associations between the traditional plasma parameters and cardiovascular events and mortality [5]. So far, clinical trials aimed at correcting biochemical abnormalities have not proven beneficial effects on hard endpoints [6]. Therefore, it is crucial to identify other pathophysiologically relevant biochemical factors with the potential for therapeutic manipulation.
Activin A is a hormone belonging to the transforming growth factor-β (TGF-β) superfamily. It is closely involved in several physiological processes including organogenesis and fibrosis. Recently, activin A has been linked to kidney disease and CKD-MBD. A few human studies have found elevated activin A plasma levels in patients with CKD [7, 8]. In rodents, plasma levels of activin A are increased in acute and chronic kidney disease [9–13], potentially due to induction and systemic secretion from injured kidneys [9, 14]. Interestingly, inhibition of activin A signalling has been shown to ameliorate vascular calcification, bone disease, cardiomyopathy and progressive kidney disease in CKD mice [11, 15, 16]. Activin A mediates several biological actions in the cardiovascular system and skeleton, including stimulation of osteoclastogenesis [17, 18]. Moreover, activin A is closely linked to
the Wnt signalling system [19]. Evidence suggests that activin A increases the kidney expression and circulating levels of Wnt inhibitor Dickkopf-1 (DKK-1), which can be reversed by activin A inhibition [19]. This implies that activin A is a suitable mediator of CKD-MBD and suggests that it may also be a potential biomarker and therapeutic target of CKD-MBD.
The aim of the current study was first, to demonstrate that p-activin A rises with declining kidney function; second, to investigate whether p-activin A could be a biomarker for vascular calcification and bone mineral density (BMD); and third, to examine whether elevated p-activin A is associated with major adverse cardiovascular events (MACE) and all-cause mortality.
MATERIALS AND METHODS
Cohort
The present study used the prospective observational Copenhagen CKD Cohort, which has previously been described in detail [20]. The study included 741 patients with CKD stage 1–5ND (no dialysis) aged 30–75 years at inclusion. Participants were recruited from October 2015 to June 2017 from the outpatient clinic at the Department of Nephrology, Rigshospitalet, Copenhagen, Denmark. CKD staging was based upon Kidney Disease: Improving Global Outcomes (KDIGO) guidelines using the Chronic Kidney Disease Epidemiology Collaboration formula [21]. Exclusion criteria comprised previous kidney transplantation with a functional graft, active malignancy, pregnancy, intellectual disability, dementia and psychosis. An age- and sex-matched control group of 175 healthy individuals was recruited [22, 23]. Their inclusion criteria were 30–75 years of age with no history of cardiovascular disease, no CKD (eGFR >60 mL/min/1.73 m2 and urine albumin:creatinine ratio <30 mg/mmol), no malignancy and no other chronic diseases.
Ethics
The study was in accordance with the Declaration of Helsinki II and approved by the Danish Scientific Ethical Committee (H-3-2011-069) and the Danish Data Protection Agency (30-0840). Written informed consent was signed by all participants before inclusion.
Design
The study was designed to answer three main questions: first, to determine p-activin A levels among a large cohort of healthy individuals and patients with CKD stage 1–5ND; second, to investigate whether p-activin A could function as a biomarker for CKD-MBD as determined by an association between p-activin A and radiographic measures of vascular calcification and BMD; and third, to examine whether p-activin A is associated with MACE and all-cause mortality (Supplementary data, Fig. S1).
The primary outcome was defined as a composite of MACE and all-cause mortality. Both were also examined independently as secondary outcomes. MACE included: myocardial infarction, percutaneous coronary intervention (PCI), coronary artery bypass grafting (CABG), ischaemic stroke, carotid endarterectomy or stenting, percutaneous transluminal angioplasty (PTA) of a lower limb, artery bypass graft of lower limb and non-traumatic lower limb amputation. All-cause mortality included death from any cause. The follow-up period was from baseline blood sample until first occurring event (MACE, mortality, lost to follow-up, withdrawal of consent) or end-of follow-up. All data were retrieved from electronic medical records.
Blood analyses
All analyses were performed by the Department of Biochemistry, Rigshospitalet, Copenhagen, Denmark. Heparinized p-activin A was measured by enzyme-linked immunosorbent assay (ELISA) (DAC00B, R&D Systems, MN, USA). Intra- and inter-assay variation was 4.2% and 7.6%, respectively.
MDCT scan (vascular calcification and BMD)
Multidetector computed tomography (MDCT) scans were performed in 580 patients with CKD (320-detector CT scanner, Aquillon One, Canon Medical Systems, Japan), as described previously [20]. Vascular calcification was assessed in the coronary arteries and thoracic aorta using the Agatston scoring method [24] with commercially available software (Vitrea 6.3, Vital Images Inc., MN, USA). Calcification was defined by a distinct voxel with an attenuation threshold ≥130 Hounsfield Units [24]. Four and 45 patients were excluded due to inadequate scan quality (motion artefact, noise artefact or out of scan field) in the coronary arteries and thoracic aorta, respectively. Participants were divided into risk groups depending on Agatston score: 0 (no calcification), 1–100, 101–400 and >400 [25].
BMD was measured in thoracic vertebrates Th7–9 from the MDCT scans using a calibration phantom pad of cylindrical bone-equivalent calcium hydroxyapatite rods with different densities (0, 75 and 150 mg/cm3 plus one fat equivalent). BMD scores were measured using commercially available semi-automated software (Nvivo™, Image Analysis, KY, USA) as described previously [26]. In brief, volumetric trabecular BMD was measured in three consecutive thoracic vertebras (Th7–9). The centre of each vertebra was marked manually in three dimensions and the region of interest was placed automatically by the software with a 2–3 mm distance to the cortical bone. In case of heavy degenerative changes, fractures, bone islands and osteophytes that compromised adequate BMD measurements the vertebra was excluded, and the final BMD analysis was based on only two vertebras. The entire analysis was excluded if two vertebras were omitted or if the images were of inadequate quality (noise, motion artefacts). As according to the American College of Radiology, BMD was presented as mg/cm3 and divided into three groups of BMD >120, 80–120 and <80 mg/cm3 corresponding to normal, osteopenic and osteoporotic levels [27, 28]. Thirty-six patients were excluded due to inadequate scan quality.
Statistics
Baseline characteristics are presented as count with percentage for categorical data, or as mean with standard deviation for normally distributed data, and median with interquartile range for non-normally distributed data. Longitudinal data were log-transformed when appropriate. One-way analysis of variance with Tukey's multiple comparison test or Kruskal–Wallis test was applied for comparisons between groups. The linear correlation of activin A with (i) eGFR, (ii) coronary Agatston score, (iii) thoracic aorta Agatston score and (iv) BMD were tested using Spearman's rank correlation, with further testing of the association in adjusted models based on analyses of covariance and ordinal logistic regression with adjustment for age, sex, diabetes mellitus (DM) and eGFR.
Five-year survival and cumulative incidence of MACE and all-cause mortality were evaluated between strata of p-activin A (low: ≤100; low-medium: 101–200; medium: 201–300; high-medium: 301–400; high: >400 pg/mL) based on the Kaplan–Meier or Aalen–Johansen estimators. Hazard ratios (HRs) for the associations of p-activin A with all-cause mortality and MACE were computed in multiple Cox regression models adjusted for age, sex, DM and eGFR including subgroup analyses. All data analyses were performed in R (version 4.0.1; R Core Team 2019). A two-tailed P-value <.05 was considered statistically significant.
RESULTS
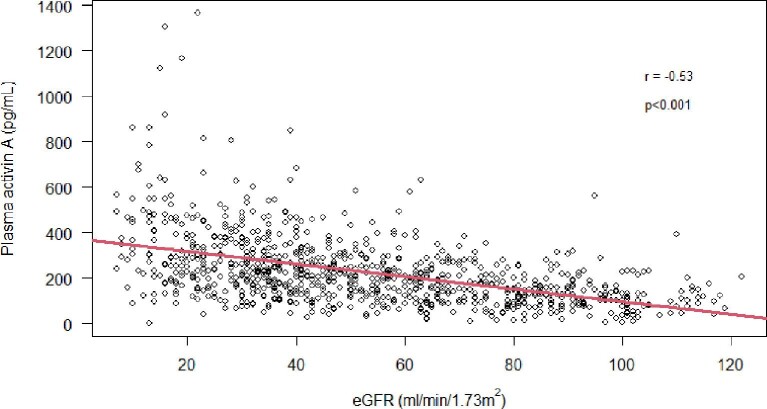
The final study cohort consisted of 916 participants (741 patients with CKD1–5ND and 175 controls). Baseline characteristics are presented in Table 1. P-activin A was measured in 896 participants (723 patients and 173 controls). Activin A was elevated in patients with CKD and rose continuously with declining kidney function, reaching statistical significance from CKD3 [control 124 (87–176) vs CKD3 225 (152–296) pg/mL, P < .001; Fig. 1]. P-activin A was inversely correlated with eGFR (Spearman r = –0.53, P < .001; Fig. 2). P-activin A was elevated irrespective of kidney disease aetiology (P < .01; Supplementary data, Table S1).
Table 1:
Baseline demographic, clinical, medical and laboratory characteristics.
| Overall | Control | CKD1 | CKD2 | CKD3 | CKD4 | CKD5ND | |
|---|---|---|---|---|---|---|---|
| N (%) | 916 | 175 (19) | 62 (7) | 115 (13) | 375 (41) | 146 (16) | 43 (5) |
| Age (years) | 61 (49–69) |
63 (52–69) |
41 (35–51) |
49 (40–62) |
64 (53–70) |
64 (54–71) |
65 (50–70) |
| Sex, female (n, %) |
354 (39) | 68 (39) | 26 (42) | 51 (44) | 133 (35) | 60 (41) | 16 (37) |
| DM (n, %) |
155 (17) | 0 (0) | 1 (2) | 5 (4) | 90 (24) | 46 (32) | 13 (30) |
| Hypertension (n, %) |
695 (76) | 51 (29) | 43 (69) | 92 (80) | 337 (90) | 131 (90) | 41 (95) |
| Vit. D suppl. (n, %) |
263 (29) | 5 (3) | 16 (26) | 34 (30) | 112 (30) | 67 (46) | 29 (67) |
| Phos. Bind. (n, %) |
26 (3) | 0 (0) | 0 (0) | 1 (1) | 0 (0) | 10 (7) | 15 (35) |
| Creatinine (µmol/L) |
124 (89–176) |
82 (71–92) |
71 (65–80) |
92 (82–102) |
141 (122–162) |
236 (206–262) |
424 (369–473) |
| eGFR (mL/min/1.73 m2) |
49 (32–74) |
81 (73–91) |
101 (95–108) |
68 (64–79) |
41 (35–50) |
22 (19–26) |
12 (10–13) |
| Haemoglobin (mmol/L) |
8.6 (7.9–9.3) |
9.0 (8.6–9.5) |
8.8 (8.1–9.4) |
8.7 (8.2–9.5) |
8.6 (8.0–9.3) |
7.9 (7.2–8.5) |
7.2 (6.8–8.0) |
| Ionized calcium (mmol/L) |
1.22 (1.19–1.25) |
1.23 (1.20–1.25) |
1.23 (1.19–1.25) |
1.23 (1.21–1.26) |
1.21 (1.19–1.25) |
1.20 (1.16–1.23) |
1.17 (1.13–1.22) |
| Phosphate (mmol/L) |
1.04 (0.91–1.81) |
1.02 (0.86–1.14) |
0.97 (0.85–1.11) |
1.02 (0.90–1.10) |
1.00 (0.89–1.15) |
1.18 (1.06–1.37) |
1.58 (1.41–1.78) |
| PTH (pmol/L) |
7 (5–10) | 5 (4–6) | 4 (4–5) | 5 (4–6) | 7 (5–10) | 13 (9–20) | 29 (16–41) |
| Activin A (pg/mL) |
198 (125–289) |
124 (87–176) |
133 (87–174) |
161 (94–222) |
225 (152–296) |
285 (197–403) |
374 (257–496) |
| BMD (mg/cm3) |
132 (102–167) |
NA | 147 (131–186) |
147 (105–184) |
126 (97–164) |
122 (97–156) |
138 (111–171) |
Values are presented as n (%) or median (interquartile range).
NA: not assessed; Vit. D suppl.: vitamin D supplements; Phos. Bind.: phosphate binders.
Figure 1:
P-activin A levels in controls and patients with CKD. P-activin A increases with declining kidney function reaching statistical significance from CKD stage 3. Groups with different letters are significantly different. P-activin A levels are presented in Table 1.
Figure 2:
Correlation between p-activin A and eGFR. P-activin A is negatively correlated with eGFR (Spearman's r = –0.53 and P < .001).
The presence of vascular calcification was determined in coronary arteries and thoracic aorta in 580 of the patients with CKD. In the crude analysis p-activin A was elevated in patients with coronary Agatston score >400 (P < .001) and in patients with thoracic aorta Agatston score of 101–400 (P < .05) and >400 (P < .001) when compared with patients having a score of 0 (Table 2). However, when adjusting for eGFR all significance was lost and no association could be demonstrated between p-activin A and Agatston score.
Table 2:
P-activin A levels in relation to vascular calcification and BMD.
| Agatston score | 0 | 1–100 | 101–400 | >400 |
|---|---|---|---|---|
| Coronary arteries | 196 (118–262) | 239 (157–337) | 226 (154–316) | 267 (187–363)***,a |
| Thoracic aorta | 196 (121–264) | 203 (142–297) | 233 (164–302)*,a | 255 (173–363)***,a |
| BMD (mg/cm3) | Normal >120 |
Osteopenic 80–120 |
Osteoporotic <80 |
|
| 210 (134–284) | 222 (154–332) | 203 (141–320) | ||
Values are presented as median (interquartile range) p-activin A in pg/mL.
*P < 0.05 and ***P < 0.001, both compared with Agatston score of 0.
aInsignificant when adjusting for eGFR.
BMD tended to decrease with advancing CKD stage reaching significance from CKD1 to 3 and 4 (P < .01; Table 1). No differences were observed between any other groups. No difference in p-activin A was detected between patients with CKD divided into groups of normal, osteopenic and osteoporotic BMD levels (Table 2).
The outcome analyses included the 896 participants with available p-activin A measurement. Median follow-up time was 4.36 (interquartile range 3.64–4.75) years. A total of 188 events occurred: 21 myocardial infarctions, 12 PCIs, 6 CABGs, 28 ischaemic strokes, 1 carotid endarterectomies/-stents, 11 PTAs of lower limb arteries, 2 artery bypass grafts of lower limb arteries, 10 non-traumatic lower limb amputations and 97 deaths. This led to 157 first events, 97 deaths and 77 MACEs for the final analyses. Six were lost to follow-up, 76 started dialysis and 30 received a kidney transplant—all included in the final analyses.
Elevated p-activin A level was associated with an increased risk of the primary outcome and both secondary outcomes when looking at p-activin A divided into strata (log-ranks P < .0001, Gray's test P < .001; Figs 3 and 4, Supplementary data, Fig. S2). Cox proportional hazard models revealed that p-activin A was associated with the composite outcome of MACE and all-cause mortality [HR 2.44 (95% confidence interval 1.88; 3.17), P < .001] as well as all-cause mortality [HR 3.13 (2.23; 4.39), P < .001] in the unadjusted analysis. However, when adjusting for age, sex, DM and eGFR only the association with all-cause mortality remained significant [HR 1.55 (1.04; 2.32), P < .05; Table 3]. Subgroup analyses showed interaction with CKD2–3 patients (P < .05; Table 4). P-activin A was associated with the secondary outcome of MACE without all-cause mortality in the unadjusted analysis [HR 2.28 (1.57; 3.29), P < .001] and when adjusted for age, sex and DM [HR 1.68 (1.13; 2.50), P < .05]. However, significance was lost when adding adjustment for eGFR [HR 1.26 (0.83; 1.91), P = .28].
Figure 3:
Kaplan–Meier curve showing unadjusted absolute risk of the primary composite outcome (MACE and all-cause mortality) evaluated between strata of p-activin A. P-activin A strata: low: <100; low-medium: 100–200; medium: 200–300; high-medium: 300–400; high: >400 pg/mL. Log-rank P < .0001.
Figure 4:
Kaplan–Meier curve showing unadjusted absolute risk of the secondary outcome all-cause mortality evaluated between strata of p-activin A. P-activin A strata: low: <100; low-medium: 100–200; medium: 200–300; high-medium: 300–400; high: >400 pg/mL. Log-rank P < .0001.
Table 3:
Adjusted and unadjusted HRs for the primary composite outcome (MACE and all-cause mortality) and the secondary outcome (all-cause mortality).
| Model | Primary composite outcome (MACE + all-cause mortality) |
Secondary outcome (all-cause mortality) |
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Unadjusted | 2.44 (1.88; 3.17) | <.001 | 3.13 (2.23; 4.39) | <.001 |
| Adjusted for age and sex | 2.05 (1.55; 2.73) | <.001 | 2.70 (1.85; 3.94) | <.001 |
| Adjusted for age, sex and DM | 1.76 (1.32; 2.34) | <.001 | 2.28 (1.55; 3.34) | <.001 |
| Adjusted for age, sex, DM and eGFR | 1.27 (0.94; 1.70) | .12 | 1.55 (1.04; 2.32) | <.05 |
Table 4:
Subgroup analysis of the primary composite outcome (MACE and all-cause mortality) and the secondary outcome (all-cause mortality).
| Primary composite outcome (MACE + all-cause mortality) |
Secondary outcome (all-cause mortality) |
||||
|---|---|---|---|---|---|
| Subgroup | N | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Sex | |||||
| Male | 560 | 1.23 (0.84; 1.82) | .29 | 1.36 (0.81; 2.28) | .24 |
| Female | 336 | 1.26 (0.80; 2.00) | .31 | 1.78 (0.94; 3.37) | .08 |
| Age | |||||
| <60 years | 428 | 1.49 (0.90; 2.47) | .12 | 1.92 (0.86; 4.26) | .11 |
| 60–70 years | 270 | 1.25 (0.72; 2.16) | .42 | 1.69 (0.83; 3.43) | .15 |
| >70 years | 198 | 1.16 (0.69; 1.96) | .57 | 1.31 (0.71; 2.45) | .39 |
| CKD stage | |||||
| 0–1 | 234 | 0.75 (0.26; 2.13) | .36 | 0.57 (0.17; 1.93) | .36 |
| 2–3 | 474 | 1.43 (0.92; 2.23) | .11 | 2.56 (1.29; 5.10) | <.01 |
| 4–5 | 188 | 1.37 (0.87; 2.15) | .42 | 1.24 (0.73; 2.08) | .42 |
| DM | |||||
| No | 747 | 1.23 (0.85; 1.77) | .27 | 1.55 (0.92; 2.59) | .10 |
| Yes | 149 | 1.33 (0.79; 2.22) | .28 | 1.50 (0.80; 2.82) | .21 |
DISCUSSION
This prospective cohort study of 916 participants, including both CKD1–5ND patients and healthy controls, is the largest to demonstrate that p-activin A increases with declining kidney function. It corroborates findings from a French and an American study [7, 29]. The French study included 232 patients with CKD2–5 and found the same association and similar p-activin A levels using an identical assay [29]. The American study investigated 104 participants with CKD2–5D including 19 controls and found the same significant relation between p-activin A and kidney function, but with a plasma level 1.5–2 times higher than that in our study. This might be due to the use of heparinized plasma versus serum for activin A measurement or be due to different ELISA kits (catalogue number not specified).
As activin A has a size of 26 kDa and therefore is expected to be freely filtered in the glomerulus, the increased plasma levels in kidney disease might just be due to accumulation. However, kidney expression and urinary excretion of activin A is undetectable in healthy individuals whereas they both increase significantly in kidney disease [30, 31]. Moreover, in rats with kidney disease, activin A levels are higher in plasma from kidney vein compared with plasma from kidney artery—as opposed to identical levels in controls [14]. Although accumulation cannot be completely ruled out, this indicates production and systemic secretion of activin A from injured kidneys. Also, other organs like the cardiovascular and inflammatory system might contribute to the increased plasma levels [29, 32, 33].
This is the first study to demonstrate that p-activin A is independently associated with all-cause mortality in a large longitudinal CKD cohort. Our findings corroborate a Norwegian study that found serum activin A to be independently associated with the composite outcome of mortality (17 events) and MACE (8 myocardial infarctions, 8 stroke, 3 hospitalization for unstable angina pectoris) in a cohort of 135 patients with type 2 DM [34]. The same group has found circulating activin A to be associated with the severity of coronary atherosclerotic burden in patients with type 2 DM [35]. Others have found increased activin A in DM [36]. We found no differences in p-activin A values among various kidney disease aetiologies. In our study, p-activin A was associated with all-cause mortality independently of DM and eGFR, but the association with MACE disappeared when adjusting for eGFR. Importantly, patients with CKD exhibit vascular calcification of both the intimal and medial arterial vessel wall. Intimal calcification is associated with older age, hypertension, diabetes, smoking, obesity, etc., and with atherosclerotic plaques and thrombotic events, whereas medial calcification is more specifically related to CKD-MBD with associated vascular stiffness, heart failure, arrythmias and sudden cardiac death [37]. The definition of MACE in the present study is primarily consistent with atherosclerotic cardiovascular events and hence mostly related to intimal calcification outcomes. It could have been interesting to investigate hospitalization from heart failure, which is more related to outcomes associated with medial calcification. In that aspect, others have found p-activin A to be elevated in patients with heart failure [32] as well as correlated with cardiac left ventricular mass/end diastolic volume ratio and aortic pulse wave velocity [38], indicating a relation to cardiac function and vascular stiffness.
Activin A signalling inhibition has been shown to ameliorate CKD-MBD-associated complications in CKD mice, where a ligand trap for the activin receptor reduced aortic calcification [11, 15]. The same ligand trap dose-dependently amended progression of vascular calcification (i.e. attenuated increase of Agatston score) in the abdominal aorta of haemodialysis patients [39]. We speculated whether p-activin A could be a biomarker of CKD-MBD complications and measured vascular calcification by coronary and thoracic aorta Agatston scores from MDCT scans and used the same scans to determine the volumetric thoracic BMD in patients with CKD. P-activin A was significantly elevated in those with the highest Agatston scores, but the association was lost when adjusting for eGFR. Indeed, Agatston score is one way to determine vascular calcium content and it is possible that other scoring systems (like the volume score or calibrated mass score) [26, 40] might have revealed different results. Together, our study does not indicate p-activin A to be a biomarker of vascular calcification in CKD.
Volumetric thoracic BMD has been shown suitable for assessing fracture risk in an adult population with eGFR >40 mL/min/1.73 m2 [27] and low levels are associated with coronary artery calcification progression and mortality [41–43]. Vascular calcification should not affect the BMD measurements assessed using MDCT scans. We found that BMD tended to decrease with declining kidney function. CKD3–4, but not 5, had significantly lower BMD levels than CKD stage 1. The BMD measurement method used in the current study determines trabecular BMD which might not be the ideal assessment of bone volume in patients with CKD. Other studies have shown that patients with CKD exhibit rapid cortical bone loss with better preservation of trabecular bone [44, 45], probably due to secondary hyperparathyroidism with PTH’s catabolic effect on cortical bone and anabolic effect on trabecular bone [1]. The present study found p-activin A to be similar among patients with CKD grouped according to categories of normal, osteopenic and osteoporotic BMD, thereby refuting p-activin A as a biomarker of BMD. In CKD-MBD, bone quality should optimally be assessed using the TMV classification (turnover–mineralization–volume) in bone biopsies [1]. Lima et al. have previously found an association between p-activin A and bone turnover in patients with CKD [7]. Hence, activin A may still be related to bone quality, but not volumetric BMD as presented in this study. One might also argue that cortical volume would be of equal interest in the evaluation of bone quality in CKD-MBD, but this could not be measured with the methods at hand. Dual energy X-ray absorptiometry (DXA) is another accepted method for assessing bone quality. DXA was introduced to the KDIGO guidelines in 2016 and relies on prospective studies showing that DXA can predict fractures in patients with CKD stage 3–5D. Future studies on CKD-MBD should ideally examine DXA and/or TMV parameters on bone biopsies as well as the association between volumetric thoracic BMD and fracture risk. Unfortunately, detailed fracture follow-up data were not available in the present cohort and bone biopsies for turnover and mineralization analyses were not taken.
The strengths of the present study were the large number of included patients and controls, as well as the longitudinal design with >4 years of follow-up. Limitations include the inability of the MDCT scans to discriminate between intimal and medial calcification. Inclusion of hospitalization for heart failure in the MACE definition might have revealed complications of medial calcification which could be more related to p-activin A than atherosclerotic events. Another limitation is the lack of validation of volumetric thoracic BMD as a predictor of fracture risk in patients with CKD. Finally, the patients who progressed to end-stage kidney disease and received dialysis or a kidney transplant were included in the follow-up analysis. Both events modify the risk of MACE and mortality, which might have influenced the results.
In conclusion, this is the largest study to show that p-activin A increases with declining eGFR and that p-activin A is associated with all-cause mortality independently of age, sex, DM and eGFR. Although activin A is implicated in CKD-MBD pathophysiology, our study opposes p-activin A as a biomarker of CKD-MBD regarding radiographic assessment of vascular calcification and BMD.
Supplementary Material
ACKNOWLEDGEMENTS
Special thanks to technicians Ida-Mari Henriksen and Charlotte Wandel for their tremendous support regarding the biobank and activin A measurements.
Contributor Information
Anders Nordholm, Department of Nephrology, Rigshospitalet, Copenhagen, Denmark; Department of Nephrology, Herlev & Gentofte Hospital, Copenhagen, Denmark.
Ida M H Sørensen, Department of Nephrology, Rigshospitalet, Copenhagen, Denmark.
Sasha S Bjergfelt, Department of Nephrology, Rigshospitalet, Copenhagen, Denmark; Department of Biomedical Sciences, University of Copenhagen, Copenhagen, Denmark.
Andreas Fuchs, Department of Cardiology, Rigshospitalet, Copenhagen, Denmark.
Klaus F Kofoed, Department of Cardiology, Rigshospitalet, Copenhagen, Denmark; Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark.
Nino E Landler, Department of Cardiology, Herlev & Gentofte Hospital, Copenhagen, Denmark.
Tor Biering-Sørensen, Department of Biomedical Sciences, University of Copenhagen, Copenhagen, Denmark; Department of Cardiology, Herlev & Gentofte Hospital, Copenhagen, Denmark.
Nicholas Carlson, Department of Nephrology, Rigshospitalet, Copenhagen, Denmark.
Bo Feldt-Rasmussen, Department of Nephrology, Rigshospitalet, Copenhagen, Denmark; Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark.
Christina Christoffersen, Department of Biomedical Sciences, University of Copenhagen, Copenhagen, Denmark; Department of Clinical Biochemistry, Rigshospitalet, Copenhagen, Denmark.
Susanne Bro, Department of Nephrology, Rigshospitalet, Copenhagen, Denmark.
Funding
This study was supported by the Augustinus Foundation, Novo Nordisk Foundation, and The Capital Region of Denmark.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
A.N. has previously owned private shares in Novo and Lundbeck. All other authors have no conflicts of interest.
REFERENCES
- 1. Moe S, Drüeke T, Cunningham J et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2006;69:1945–53. 10.1038/sj.ki.5000414 [DOI] [PubMed] [Google Scholar]
- 2. Go AS, Chertow GM, Fan D et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–305. 10.1056/nejmoa041031 [DOI] [PubMed] [Google Scholar]
- 3. Smith ER, Hewitson TD, Holt SG. Diagnostic tests for vascular calcification. Adv Chronic Kidney Dis 2019;26:445–63. 10.1053/J.ACKD.2019.07.001 [DOI] [PubMed] [Google Scholar]
- 4. Bover J, Ureña-Torres P, Cozzolino M et al. The non-invasive diagnosis of bone disorders in CKD. Calcif Tissue Int 2021;108:512–27. 10.1007/S00223-020-00781-5/TABLES/3 [DOI] [PubMed] [Google Scholar]
- 5. Lunyera J, Scialla JJ. Update on chronic kidney disease mineral and bone disorder in cardiovascular disease. Semin Nephrol 2018;38:542–58. 10.1016/j.semnephrol.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ketteler M, Block GA, Evenepoel P et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: what's changed and why it matters. Kidney Int 2017;92:26–36. 10.1016/J.KINT.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 7. Lima F, Mawad H, El-Husseini AA et al. Serum bone markers in ROD patients across the spectrum of decreases in GFR: activin A increases before all other markers. Clin Nephrol 2019;91:222–30. 10.5414/CN109650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maeshima A, Mishima K, Yamashita S et al. Follistatin, an activin antagonist, ameliorates renal interstitial fibrosis in a rat model of unilateral ureteral obstruction. Biomed Res Int 2014;2014:37619. 10.1155/2014/376191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nordholm A, Mace ML, Gravesen E et al. Klotho and activin a in kidney injury: plasma Klotho is maintained in unilateral obstruction despite no upregulation of Klotho biosynthesis in the contralateral kidney. Am J Physiol Renal Physiol 2018;314:F753–62. 10.1152/ajprenal.00528.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nordholm A, Egstrand S, Gravesen E et al. Circadian rhythm of activin A and related parameters of mineral metabolism in normal and uremic rats. Pflugers Arch 2019;471:1079–94. 10.1007/s00424-019-02291-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Agapova OA, Fang Y, Sugatani T et al. Ligand trap for the activin type IIA receptor protects against vascular disease and renal fibrosis in mice with chronic kidney disease. Kidney Int 2016;89:1231–43. 10.1016/j.kint.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hortells L, Sosa C, Guillén N et al. Identifying early pathogenic events during vascular calcification in uremic rats. Kidney Int 2017;92:1384–94. 10.1016/j.kint.2017.06.019 [DOI] [PubMed] [Google Scholar]
- 13. Solagna F, Tezze C, Lindenmeyer MT et al. Pro-cachectic factors link experimental and human chronic kidney disease to skeletal muscle wasting programs. J Clin Invest 2021;131:e135821. 10.1172/JCI135821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Egstrand S, Olgaard K, Lewin E. Circadian rhythms of mineral metabolism in chronic kidney disease-mineral bone disorder. Curr Opin Nephrol Hypertens 2020;29:367–77. 10.1097/MNH.0000000000000611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williams MJ, Sugatani T, Agapova OA et al. The activin receptor is stimulated in the skeleton, vasculature, heart, and kidney during chronic kidney disease. Kidney Int 2018;93:147–58. 10.1016/j.kint.2017.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sugatani T, Agapova OA, Fang Y et al. Ligand trap of the activin receptor type IIA inhibits osteoclast stimulation of bone remodeling in diabetic mice with chronic kidney disease. Kidney Int 2017;91:86–95. 10.1016/j.kint.2016.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sugatani T. Systemic activation of activin a signaling causes chronic kidney disease-mineral bone disorder. Int J Mol Sci 2018;19:2490. 10.3390/ijms19092490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bloise E, Ciarmela P, Dela Cruz C et al. Activin A in mammalian physiology. Physiol Rev 2019;99:739–80. 10.1152/physrev.00002.2018 [DOI] [PubMed] [Google Scholar]
- 19. Cianciolo G, La Manna G, Capelli I et al. The role of activin: the other side of chronic kidney disease–mineral bone disorder? Nephrol Dial Transplant 2021;36:966–74. 10.1093/ndt/gfaa203 [DOI] [PubMed] [Google Scholar]
- 20. Sørensen IMH, Saurbrey SAK, Hjortkjær HØ et al. Regional distribution and severity of arterial calcification in patients with chronic kidney disease stages 1–5: a cross-sectional study of the Copenhagen chronic kidney disease cohort. BMC Nephrol 2020;21:534. 10.1186/S12882-020-02192-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sørensen IM, Bisgaard LS, Bjergfelt SS et al. The metabolic signature of cardiovascular disease and arterial calcification in patients with chronic kidney disease. Atherosclerosis 2022;350:109–18. 10.1016/J.ATHEROSCLEROSIS.2022.03.019 [DOI] [PubMed] [Google Scholar]
- 23. Bjergfelt SS, Sørensen IMH, Hjortkjær H et al. Carotid plaque thickness is increased in chronic kidney disease and associated with carotid and coronary calcification. PLoS One 2021;16:e0260417. 10.1371/JOURNAL.PONE.0260417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agatston AS, Janowitz WR, Hildner FJ et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–32. 10.1016/0735-1097(90)90282-T [DOI] [PubMed] [Google Scholar]
- 25. Erbel R, Mhlenkamp S, Moebus S et al. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol 2010;56:1397–406. 10.1016/J.JACC.2010.06.030 [DOI] [PubMed] [Google Scholar]
- 26. Wiegandt YL, Sigvardsen PE, Sørgaard MH et al. The relationship between volumetric thoracic bone mineral density and coronary calcification in men and women - results from the Copenhagen General Population Study. Bone 2019;121:116–20. 10.1016/J.BONE.2019.01.010 [DOI] [PubMed] [Google Scholar]
- 27. Therkildsen J, Nissen L, Jørgensen HS et al. Thoracic bone mineral density derived from cardiac CT is associated with greater fracture rate. Radiology 2020;296:499–508. 10.1148/RADIOL.2020192706 [DOI] [PubMed] [Google Scholar]
- 28. American College of Radiology (ACR) . Practice Parameter for the Performance of Quantitative Computed Tomography (QCT) Bone Densitometry. Revised 2023. https://www.acr.org/-/media/ACR/Files/Practice-Parameters/QCT.pdf (28 September 2023, date last accessed). [Google Scholar]
- 29. Bataille S, Dou L, Bartoli M et al. Mechanisms of myostatin and activin A accumulation in chronic kidney disease. Nephrol Dial Transplant 2022;37:1249–60. 10.1093/NDT/GFAC136 [DOI] [PubMed] [Google Scholar]
- 30. Iriuchishima H, Maeshima A, Takahashi S et al. Activin A: a novel urinary biomarker of renal impairment in multiple myeloma. Biosci Rep 2019;39:BSR20190206. 10.1042/BSR20190206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takahashi S, Nakasatomi M, Takei Y et al. Identification of urinary activin A as a novel biomarker reflecting the severity of acute kidney injury. Sci Rep 2018;8:5176. 10.1038/s41598-018-23564-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yndestad A, Ueland T, Øie E et al. Elevated levels of activin A in heart failure: potential role in myocardial remodeling. Circulation 2004;109:1379–85. 10.1161/01.CIR.0000120704.97934.41 [DOI] [PubMed] [Google Scholar]
- 33. Chen W, Ten Dijke P. Immunoregulation by members of the TGFβ superfamily. Nat Rev Immunol 2016;16:723–40. 10.1038/nri.2016.112 [DOI] [PubMed] [Google Scholar]
- 34. Ofstad AP, Gullestad L, Orvik E et al. Interleukin-6 and activin A are independently associated with cardiovascular events and mortality in type 2 diabetes: the prospective Asker and Bærum Cardiovascular Diabetes (ABCD) cohort study. Cardiovasc Diabetol 2013;12:126. 10.1186/1475-2840-12-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ueland T, Aukrust P, Aakhus S et al. Activin A and cardiovascular disease in type 2 diabetes mellitus. Diab Vasc Dis Res 2012;9:234–7. 10.1177/1479164111431171/ASSET/IMAGES/LARGE/10.1177_1479164111431171-FIG1.JPEG [DOI] [PubMed] [Google Scholar]
- 36. Kuo CS, Lu YW, Hsu CY et al. Increased activin A levels in prediabetes and association with carotid intima-media thickness: a cross-sectional analysis from I-Lan Longitudinal Aging Study. Sci Rep 2018;8:1–9. 10.1038/s41598-018-27795-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goodman WG, London G. Vascular calcification in chronic kidney disease. Am J Kidney Dis 2004;43:572–9. 10.1053/j.ajkd.2003.12.005 [DOI] [PubMed] [Google Scholar]
- 38. Chen WJY, Greulich S, van der Meer RW et al. Activin a is associated with impaired myocardial glucose metabolism and left ventricular remodeling in patients with uncomplicated type 2 diabetes. Cardiovasc Diabetol 2013;12:150. 10.1186/1475-2840-12-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Coyne DW, Singh HN, Smith WT et al. Sotatercept safety and effects on hemoglobin, bone, and vascular calcification. Kidney Int Rep 2019;4:1585–97. 10.1016/j.ekir.2019.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Callister TQ, Cooil B, Raya SP et al. Coronary artery disease: improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology 1998;208:807–14. 10.1148/RADIOLOGY.208.3.9722864 [DOI] [PubMed] [Google Scholar]
- 41. Watanabe R, Lemos MM, Carvalho AB et al. The association between coronary artery calcification progression and loss of bone density in non-dialyzed CKD patients. Clin Nephrol 2012;78:425–31. 10.5414/CN107515 [DOI] [PubMed] [Google Scholar]
- 42. Filgueira A, Carvalho AB, Tomiyama C et al. Is coronary artery calcification associated withvertebral bone density in nondialyzed chronic kidney disease patients? Clin J Am Soc Nephrol 2011;6:1456–62. 10.2215/CJN.10061110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen Z, Qureshi AR, Ripsweden J et al. Vertebral bone density associates with coronary artery calcification and is an independent predictor of poor outcome in end-stage renal disease patients. Bone 2016;92:50–7. 10.1016/J.BONE.2016.08.007 [DOI] [PubMed] [Google Scholar]
- 44. Sharma AK, Toussaint ND, Masterson R et al. Deterioration of cortical bone microarchitecture: critical component of renal osteodystrophy evaluation. Am J Nephrol 2018;47:376–84. 10.1159/000489671 [DOI] [PubMed] [Google Scholar]
- 45. Nickolas TL, Stein EM, Dworakowski E et al. Rapid cortical bone loss in patients with chronic kidney disease. J Bone Miner Res 2013;28:1811–20. 10.1002/JBMR.1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.