ABSTRACT
Atherosclerotic renovascular disease (ARVD) is the most common type of renal artery stenosis. It represents a common health problem with clinical presentations relevant to many medical specialties and carries a high risk for future cardiovascular and renal events, as well as overall mortality. The available evidence regarding the management of ARVD is conflicting. Randomized controlled trials failed to demonstrate superiority of percutaneous transluminal renal artery angioplasty (PTRA) with or without stenting in addition to standard medical therapy compared with medical therapy alone in lowering blood pressure levels or preventing adverse renal and cardiovascular outcomes in patients with ARVD, but they carried several limitations and met important criticism. Observational studies showed that PTRA is associated with future cardiorenal benefits in patients presenting with high-risk ARVD phenotypes (i.e. flash pulmonary oedema, resistant hypertension or rapid loss of kidney function). This clinical practice document, prepared by experts from the European Renal Best Practice (ERBP) board of the European Renal Association (ERA) and from the Working Group on Hypertension and the Kidney of the European Society of Hypertension (ESH), summarizes current knowledge in epidemiology, pathophysiology and diagnostic assessment of ARVD and presents, following a systematic literature review, key evidence relevant to treatment, with an aim to support clinicians in decision making and everyday management of patients with this condition.
Keywords: angioplasty, atherosclerotic renovascular disease, epidemiology, renal artery stenosis, revascularization
INTRODUCTION
Atherosclerotic renovascular disease (ARVD) represents the most common type of renal artery stenosis (RAS), accounting for ≈90% of patients with RAS versus 10% of cases that are due to fibromuscular dysplasia (FMD) and other, more rare causes [1–4]. ARVD is commonly clustered with various comorbidities, including hypertension, chronic kidney disease (CKD), peripheral artery disease, coronary artery disease and heart failure (HF), and its overall prognosis is unfavourable [5]. Despite the fact that the pathophysiological basis of RAS was originally described almost 100 years ago [6, 7], the optimal treatment of ARVD is still highly controversial [8]. Preliminary observational evidence indicated the efficacy and safety of percutaneous transluminal renal artery angioplasty (PTRA) with or without stenting for the treatment of ARVD, but subsequent randomized controlled trials failed to demonstrate the superiority of revascularization in addition to standard medical therapy compared with medical therapy alone in lowering blood pressure (BP) levels or preventing adverse cardiovascular (CV) and renal outcomes in patients with ARVD [9–11]. However, these trials had several limitations in design or execution flaws and met important criticism [2]. Recent observational studies in ARVD patients with high-risk clinical presentations have demonstrated that successful restoration of blood flow is associated with the preservation of kidney function and decreased risk of CV events and death [8].
This document was prepared by experts from the European Renal Best Practice (ERBP) board of the European Renal Association (ERA) and from the Working Group on Hypertension and the Kidney of the European Society of Hypertension (ESH). It briefly summarizes current knowledge in epidemiology, pathophysiology and diagnostic assessment of ARVD and presents, following a systematic literature review, key evidence relevant to medical or interventional treatment, with an aim to support clinicians in decision -making and everyday management of patients with this condition.
Epidemiology
Prevalence
The prevalence of ARVD differs considerably among studied populations. In a population-based cohort of 870 participants >65 years of age, ARVD (defined as >60% stenosis) was identified by renal duplex sonography in 6.8% of patients, with equal frequency among White and Black participants [12]. Among hypertensives, the prevalence of ARVD is probably ≈1% in patients with mild hypertension [13], but may be as high as 14–24% in patients with severe or resistant hypertension [14–16]. Of note, among patients with hypertension, ARVD is considered the second most common cause of secondary hypertension [17].
The prevalence of ARVD is higher in individuals with atherosclerotic lesions in other vascular beds [18]. It has been documented that ARVD prevalence ranges from 12 to 45% of patients with peripheral artery disease (PAD) and from 5 to 30% in patients evaluated for coronary artery disease (CAD) [18, 19]. Studies in patients with abdominal aortic aneurysm (AAA) report prevalence rates of ARVD ranging from 2.6 to 30% depending on the criteria used for case definitions [18, 20, 21]. Finally, a relevant study reported that 54% of patients with HF and ejection fraction (EF) <40% had RAS of >50% of the luminal diameter as assessed by magnetic resonance angiography (MRA), while in the subgroup of patients with HF and CKD, the prevalence increased to 68% [22].
The association of ARVD with CKD is well described; the kidney disease resulting from stenotic lesions in the renal arteries is termed ischaemic nephropathy [23]. In middle-aged patients with advanced CKD, the prevalence of ARVD ranges from 5 to 22%, while in dialysis patients it can go as high as 40.8% [24–28]. A previous report from the US Renal Data System suggested a gradual increase in the diagnosis of ARVD from 9.2% to 11.2% in the 2-year period before dialysis initiation, based on relevant claims [29].
Prognosis
The overall prognosis of ARVD is poor. In an analysis of a 5% random sample of the US Medicare population, adverse event rates after incident diagnosis of ARVD greatly exceeded those in the general population, including atherosclerotic heart disease (303.9 versus 73.5 per 1000 patient-years), PAD (258.6 versus 52.2 per 1000 patient-years), congestive HF (CHF; 194.5 versus 56.3 per 1000 patient-years); cerebrovascular accident or transient ischaemic attack (175.5 versus 52.9 per 1000 patient-years), death (166.3 versus 63.3 per 1000 patient-years) and renal replacement therapy (28.8 versus 1.3 per 1000 patient-years) [30]. Patients with ARVD and pre-dialysis CKD have 1.5 times and those on dialysis 3.3 times higher mortality risk than patients with other causes of CKD [31]. RAS prognosis varies considerably depending on the underlying clinical presentation and comorbid conditions. A prospective cohort study in 467 patients with RAS >50% showed that among patients not treated with revascularization, those that present with flash pulmonary oedema have a markedly increased risk of death {hazard ratio [HR] 2.2 [95% confidence interval (CI) 1.4–3.5]} and CV events [HR 3.1 (95% CI 1.7–5.5)] compared with patients with low-risk phenotypes (i.e. those without flash pulmonary oedema, refractory hypertension or rapid loss of kidney function) [32].
Pathophysiology
The pathogenesis of BP rise in RAS was first described 90 years ago [7]. Currently it is widely accepted that ARVD with lumen stenosis >70% will cause a reduction of renal blood flow and hypoperfusion of the juxtaglomerular apparatus, which in turn stimulates the release of renin followed by increased production of angiotensin II and aldosterone [1, 2, 19, 33]. Stenoses of lesser degrees are suggested to have minimal haemodynamic effects due to the compensatory mechanisms of renal autoregulation [1, 19]. However, renal autoregulation may be compromised in several groups of individuals, including the elderly and those with diabetes mellitus or HF [34, 35], and thus it cannot be excluded that renal blood flow in such patient groups could be compromised in stenoses of lesser degrees. Furthermore, ischaemia of even a few nephrons can cause the full syndrome of renovascular hypertension, as shown in numerous cases of segmental RAS with excess unilateral renin release, striking hyperplasia of the juxtaglomerular apparatus of affected glomeruli and reversal of hypertension by partial nephrectomy or angiotensin-converting enzyme inhibitor (ACEI) use [36–38]. In unilateral RAS, the contralateral healthy kidney is expected to, at least partly, compensate for the above adverse effects of renin-dependent hypertension through pressure natriuresis. However, in the increasingly common cases of bilateral RAS, this compensation cannot be present, causing sodium/volume retention and leading to a volume-dependent hypertension phenotype and higher risk of ‘flash’ pulmonary oedema [39, 40].
The compensatory increase in renin–angiotensin–aldosterone system activity will have systemic effects, including increased sympathetic activity, arterial remodelling and vasoconstriction, activation of inflammatory and oxidative stress pathways and sodium and water retention. All the above would be added to the direct effects of glomerular hypoperfusion and adversely affect renal oxygenation and mitochondrial and microvascular function, leading to kidney fibrosis [1, 2, 19, 33]. In addition to the above, excess angiotensin II and aldosterone directly affect gene regulation in cardiac myocytes and fibroblasts, leading to excess inflammation and oxidative stress, upregulation of trophic factors and cytokines, myocardial cell hypertrophy, hyperplasia and apoptosis, extracellular matrix and collagen deposition and myocardial fibrosis [41–43]. Other adverse CV effects of ARVD include atheromatous plaque formation in the aortic arch, descending thoracic aorta [44] and carotid arteries [45]; increased macrophage influx and myocyte necrosis [46]; direct cardiac mitochondrial injury and impaired mitophagy [47]. All these pathways lead to adverse left ventricle (LV) remodelling/hypertrophy and diastolic dysfunction, which can be considerably reversed by renal revascularization [40, 48].
Clinical manifestations
Patients with ARVD may present with multiple clinical manifestations, ranging from asymptomatic disease to severe presentations, including malignant hypertension, flash pulmonary oedema and rapidly progressive kidney function decline. In some cases, ARVD may be identified as an incidental finding during imaging studies. Table 1 summarizes the most common clinical presentations and changes in common laboratory tests related to ARVD that should prompt clinicians to perform further investigations for this entity. The most common clinical presentations are resistant hypertension (defined as uncontrolled hypertension under office and ambulatory conditions despite appropriate lifestyle measures and optimal treatment with adequate doses of three or more antihypertensive drugs of different classes, including a diuretic, or controlled BP in the presence of adequate doses of more than four antihypertensive drugs) [49, 50] and ischaemic nephropathy, -CKD. Other clinical presentations of ARVD include a decline in glomerular filtration rate (GFR) >30% within the first weeks of initiating an ACEI or an angiotensin II receptor blocker (ARB), unexplained rapid decline in kidney function [acute kidney injury (AKI)] [51], ‘flash’ pulmonary oedema (known as Pickering syndrome) or repeated hospitalizations for decompensated HF with preserved EF, particularly in patients with hypertension and CKD [52, 53]. In unilateral RAS, the non-stenotic kidney can have damage associated with glomerular hypertension and hyperfiltration. Clinically, this can be associated with proteinuria that may reach nephrotic levels in some patients with severe hypertension [54], and can be reversed following BP reduction after revascularization by surgery or PTRA [55–57]. Histology in the non-stenotic kidney can reveal benign nephrosclerosis, focal and segmental glomerulosclerosis or relevant types of damage [54, 57, 58]. Increased albuminuria or proteinuria is a poor predictor of renal and cardiovascular outcome in patients with RAS [59].
Table 1:
Clinical phenotypes of ARVD that should prompt investigations for this entity.
| Hypertension | Sudden onset or worsening of existing hypertensionGrade III hypertension (especially in the presence of other cardiovascular risk factors or atherosclerotic disease in other circulatory beds)Resistant hypertension |
| Kidney disease | Atrophic kidney or size difference >1.5 cm between kidneysRapid, unexplained kidney function declineDecline in kidney function (eGFR) >30% after starting treatment with ACEIs/ARBsIncreased albuminuria/proteinuria due to hypertensive damage in the non-stenotic kidney in unilateral RAS |
| Heart failure | Repeated hospital admissions for decompensated heart failure with preserved left ventricular function on echocardiographySudden unexplained (‘flash’) pulmonary oedema |
Diagnosis
Duplex ultrasound is the most commonly used screening tool for ARVD due to its lower cost and non-invasive nature [19]. It provides waveform and velocity data [peak systolic velocity (PSV) and renal:aorta PSV ratio] [60], as well as data about kidney viability (resistive index) [61, 62]. A PSV >200 cm/sec is associated with 95% sensitivity and 90% specificity for >50% stenosis; a renal:aorta PSV ratio ≥3.5 has 92% sensitivity for 60% stenosis [63]. A renal-resistive index <0.8 indicates possible viability of renal parenchyma. However, the method is largely operator dependent and may be limited by the body habitus (obesity) and presence of bowel gas. Renal scintigraphy with and without captopril can offer useful information with regards to relevant blood flow in patients with unilateral RAS, but can be difficult to interpret in bilateral RAS and does not provide information on the degree of the stenosis [64].
Computed tomography angiography (CTA) or MRA are reliable methods for diagnosing RAS [19]. In older studies, the sensitivity and specificity of CTA and MRA were shown to be 64% and 92% and 62% and 84%, respectively [65]; however, with higher resolutions offered by newer devices and updated study protocols, the sensitivity and specificity is suggested to be improved, ranging between 90–96% and 90–92% with CTA and 94–97% and 85–93% with MRA [5, 19]. Both techniques are very useful for assessment of renal branching/orientation patterns and renal accessory arteries [19]. Contrast medium–induced nephropathy is considered the major limitation of CTA, although the risk is ≈5% even in individuals with stage 4 CKD [66, 67]. MRA is a reasonable alternative in these patients, since the risk of nephrogenic systemic fibrosis patients with CKD stage 4 or 5 receiving a group II gadolinium-based contrast agent was recently reported at <0.07% [68]. Apart from evaluating the actual stenosis degree, several preliminary studies suggest that the novel technique of blood oxygen level–dependent (BOLD) magnetic resonance imaging (MRI) can efficiently detect still viable ‘hibernating’ renal parenchyma and predict the positive renal functional response to renal artery revascularization [69–71]. Future developments in this area are awaited with interest.
Despite improvements in the diagnostic accuracy of CTA and MRA, invasive catheter angiography remains the gold standard method for RAS diagnosis and evaluation and it should guide the final decision to intervene. Catheter angiography additionally enables measurement of pre- and post-intervention pressure gradients to establish the haemodynamic severity of the stenosis and the possibility of treatment in the same setting and time [19]. By expert consensus and based on studies [72, 73], an angiographic RAS >70% lumen stenosis is considered severe or significant, and stenoses of 50–70% are considered moderately severe and of uncertain haemodynamic significance [74]. For moderately severe stenoses, confirmation of the haemodynamic severity of the RAS is recommended prior to stenting [74]. A resting or hyperaemic translesional systolic gradient ≥20 mmHg, a resting or hyperaemic mean translesional gradient ≥10 mmHg or a renal fractional flow reserve (RFFR) ≤0.8 confirms haemodynamically severe RAS [74].
Management
ARVD management includes medical therapy with or without renal artery revascularization.
Medical therapy
Medical management of ARVD should aim to reduce CV risk and protect kidney function. Hypertension control is a prominent goal. Since no clinical studies examining different BP targets have been performed in patients with RAS, the general recommendations should be followed [17]. With regards to antihypertensive classes, ACEIs and ARBs are considered first-line options, as observational studies suggest that these agents can reduce mortality risk in patients with ARVD [75–77]. However, these agents should be initiated with particular care, and an estimated GFR (eGFR) decline of >30% should prompt further evaluation of the patient for revascularization, as discussed below. In cases of bilateral RAS or RAS in solitary kidneys, ACEIs and ARBs should generally be avoided and the patient evaluated directly for revascularization, as they may significantly compromise renal function and there is no relevant evidence in support of their use. Most often, several antihypertensive agents are needed to achieve the BP targets, selection of which should follow the general guidelines and include dihydropyridine calcium channel blockers (CCBs), diuretics of appropriate class and dose, β-blockers and second-line agents [17]. Lipid-lowering agents are needed to achieve cholesterol targets depending on total CV risk [78]. The role of antiplatelet therapy in mitigating adverse CV outcomes in patients with ARVD has not been tested in clinical trials, but its role is established in patients with atherosclerotic coronary or peripheral artery disease [79], and observational studies have shown that the use of low-dose aspirin is associated with reduced mortality risk in patients with ARVD [80]. Additional measures to help BP control and CV risk reduction include smoking cessation, weight loss, regular physical exercise and reduced sodium consumption [17, 19, 81]; for all these measures, no hard evidence from randomized controlled trials (RCTs) in patients with ARVD is available.
Renal artery revascularization
The evolution of evidence in the field of revascularization for ARVD has gone through different phases [8]. During the early 1990s, a relative enthusiasm towards the benefit of revascularization occurred, following observational data showing significant BP reductions and stabilization of kidney function in many individuals. In later years, a few RCTs attempted to properly test the superiority of standard medical therapy plus PTRA with or without stenting compared with medical therapy alone in lowering BP levels or preventing adverse renal and CV outcomes in patients with ARVD. They largely showed that PTRA had no additional benefit on BP control, renal function and adverse CV or renal outcomes when compared with medical therapy alone [2, 8, 82]. However, these trials met severe criticism due to numerous limitations in study design, methodology and execution, as discussed in detail below. To this end, a second round of observational cohort studies examined whether PTRA would be of benefit for patients with clinical presentation highly suggestive of functionally critical ARVD, such as those with flash pulmonary oedema, refractory hypertension or rapid loss of kidney function, and helped to establish a clearer picture for current recommendations.
Observational studies
Preliminary studies of surgical revascularization for ARVD showed significant BP reductions and stabilization of kidney function in some individuals [83–86]. With the expansion of endovascular revascularization procedures, PTRA with stent implantation was subsequently widely applied for ARVD, allowing treatment of individuals deemed to be at high surgical risk [2, 86]. Several prospective observational studies published in the late 1990s showed that PTRA with or without stenting was effective for BP reduction, with decreases in systolic BP (SBP) levels of 20–25 mmHg [87–89], and for stabilization or improvement of kidney function [87, 88, 90]. In an international registry of 265 consecutive patients with ARVD (≥50% stenosis) treated with PTRA with stenting, BP levels were reduced from 160/86 mmHg to 135/75 mmHg after a median follow-up of 23.8 months. In addition, eGFR improved in 53.9% of patients, remained unchanged in 15.5% and continued to deteriorate in 30.6%. Patients whose eGFR or BP improved or stabilized had lower pre-procedural SBP and more severe stenotic lesions at baseline (longer lesion and/or higher stenosis degree) compared with patients with worsening renal function [91].
In addition to the above, several reports of individual cases and cohorts showed that patients with high-risk presentations of AVRD (flash pulmonary oedema, refractory hypertension or rapid loss of kidney function) were those that benefit the most from revascularization. These patients often have nearly occluded renal arteries or bilateral RAS or single RAS with a solitary kidney, and revascularization had immediate beneficial effects with substantial decreases in BP and significant improvement in kidney function [2]. Following the publications of relevant RCTs that almost entirely excluded patients with ARVD and high-risk clinical presentations, as discussed in detail below, several observational cohort studies examined whether PTRA with stenting might be beneficial for these individuals.
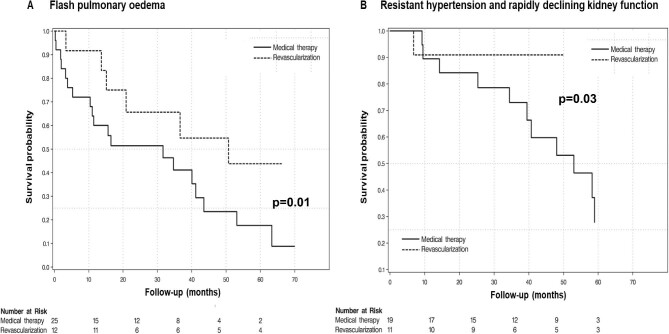
A previous prospective cohort study evaluated 467 individuals with RAS >50% who received either medical treatment or medical treatment plus PTRA with stenting in UK, and examined future CV events and mortality depending on the clinical presentation, as well as the relevant treatment. Among patients receiving only medical treatment, those who presented with flash pulmonary oedema had a markedly increased risk of death [HR 2.2 (95% CI 1.4–3.5)] and CV events [HR 3.1 (95% CI 1.7–5.5)] compared with patients with low-risk phenotypes (i.e. those without flash pulmonary oedema, refractory hypertension or rapid loss of kidney function) [32]. In the same study, PTRA with stenting compared with medical treatment was associated with large reductions in the risk of death [HR 0.15 (95% CI 0.02–0.9)] and CV events [HR 0.23 (95% CI 0.1–0.6)] in patients with high-risk presentations (Fig. 1), but no apparent benefit was observed in patients without the above high-risk presentations [HR 0.8 (95% CI 0.7–1.2) and 1.0 (95% CI 0.8–1.2), respectively]. A recent prospective cohort study investigating the effects of PTRA with stenting on ambulatory BP levels, eGFR and HF recurrence in a group of well-defined patients with severe ARVD (defined as RAS >70% in CTA or MRA and at least one of the following: resistant hypertension confirmed by 24-hour ABPM, reduction in eGFR >5 ml/min/1.73 m2/year or hospitalization for acute decompensated HF with no obvious explanation) demonstrated that PTRA was associated with better ambulatory BP levels [24-hour SBP change from baseline: −25.7 mmHg (95% CI −30.8 to −20.6)] and BP control [change in the number of antihypertensive drugs: −0.9 (95% CI −1.3 to −0.5)], improved kidney function [eGFR change: +7.2 ml/min (95% CI 3.2–11.2)] at the 24-month evaluation and reduced hospital admissions for HF/flash pulmonary oedema (of 17 patients with a history of hypertensive HF, 14 patients had no new episodes after PTRA with stenting) [92].
Figure 1:
Kaplan–Meier curves for overall mortality in individuals with ARVD and high-risk clinical phenotypes (A: flash pulmonary oedema; B: resistant hypertension and rapidly declining kidney function). Groups compared are patients having received PTRA versus those receiving medical therapy only. Modified from Ritchie et al. (with permission) [32].
RCTs
The major RCTs in the field of revascularization of ARVD are summarized in Table 2. A systematic literature search in four major databases (PubMed/MEDLINE, Cochrane/CENTRAL, Scopus and Web of Science) up to February 2023 was conducted using various combinations of the following keywords: ‘randomized controlled trial’, ‘atherosclerosis’, ‘atherosclerotic’, ‘renal artery stenosis’, ‘endovascular’, ‘angioplasty’, ‘surgical intervention’, ‘revascularization’, ‘stent’. An example of the search strategy used is presented in Supplementary Table 1. In addition, a reference search was carried out to identify additional publications by screening reference lists. Eligible studies were RCTs including adult patients with ARVD randomized to either PTRA (with or without stenting) or medical therapy. Non-randomized comparisons, observational studies or trials comparing surgical interventions with either PTRA or medical therapy were excluded. Two authors (M.T. and P.S.) independently screened the records by title, abstract and full text to identify eligible publications. The flow diagram of the study selection process is depicted in Supplementary Fig. 1.
Table 2:
Major RCTs of PTRA with and without stenting versus medical therapy in ARVD.
| Study ID | Design | N | Endpoints | Results |
|---|---|---|---|---|
| EMMAPlouin et al., 1998 | Multicentre RCTNo blinding of interventionStandardized medical treatmentFU: 6 months | 49 | Primary: mean 24-h BPSecondary: number and DDD of antihypertensive drugs, creatinine clearance, rate of occluded arteries, complications | No significant difference in ambulatory BPPTRA: fewer antihypertensive drugs (1.0 versus 1.78; P < .01), higher complication rate |
| SNRASCGWebster et al., 1998 | Multicentre RCTNo blinding of interventionStandardized medical treatmentFU: 6 months | 55 | Primary: office BP, serum creatinineSecondary: number antihypertensive drugs, complications | PTRA: significant BP reduction only if bilateral RAS; no significant difference in CV events or renal function20% participants assigned to PTRA had a surgery |
| DRASTICVan Jaarsveld et al., 2000 | Multicentre RCTNo blinding of interventionFU: 12 months | 106 | Primary: mean office BPSecondary: number and DDD of antihypertensive drugs, serum creatinine, restenosis, complications | No significant difference in SBP and DBPPTRA: fewer antihypertensive drugs (1.9 versus 2.4; P < .01)44% of participants assigned to medical therapy underwent revascularization at 3 months if DBP >95 mmHg despite three or more antihypertensive drugsOnly 3.6% stenting |
| NITERScarpioni et al., 2005*from post hoc analysis published in 2018 | Multicentre RCT, prematurely terminated | 51 | Primary: composite of all-cause mortality, CV events, dialysis | Low albuminuria group (n = 26): event-free survival 83% in PTRA versus 45% in medical therapy (P = .501)High-albuminuria group (n = 25): event-free survival 64% in PTRA versus 52% in medical therapy (P = .644) |
| STARBax et al., 2009 | Multicentre RCTNo blinding of interventionFU: 24 months | 140 | Primary: worsening of renal function (>20% decline in eCrCl with Cockcroft–Gault formula)Secondary: office BP, incidence of refractory or malignant hypertension, pulmonary oedema, CV morbidity, CV mortality, total mortality | No significant difference in renal function, BP, CV mortality and morbidity28% of participants allocated to PTRA did not undergo revascularization, mainly due to minimal stenosis1.3% crossover |
| ASTRALWheatley et al., 2009 | Multicentre RCTNo blinding of interventionMedical treatment was not standardizedMedian FU: 34 months | 806 | Primary: renal outcome (reciprocal of serum creatinine)Secondary: office BP, time to renal and major CV events and mortality, complications | No significant difference in renal function, BP, CV events and mortality17% of participants allocated to PTRA, did not undergo revascularization6% crossover |
| RASCADMarcantoni et al., 2012 | Single-centre RCTSingle-blindedStandardized medical treatmentFU: 12 months | 84 | Primary: change in echocardiographic LVMISecondary: LV function, CV events and mortality, BP control, kidney function | No significant difference in change in LVMI, BP, eGFR, CV events and mortality |
| CORALCooper et al., 2014 | Multicentre RCTNo blinding of interventionStandardized medical treatmentMedian FU: 43 months | 947 | Primary: composite of adverse fatal and non-fatal CV and renal eventsSecondary: all-cause mortality, SBP, restenosis, renal resistance index, QOL, cost-effectiveness | No significant difference in primary composite endpoint, any of individual components of primary endpoint or all-cause mortalityAlmost 17% of participants either withdrew or were lost to follow-up5.4% of participants allocated to PTRA did not undergo revascularization4% of participants allocated to medical therapy crossed over |
| RADARZeller et al., 2017 | Multicentre RCTNo blinding of interventionStandardized medical treatmentFU: 3 yearsPrematurely terminated | 86 | Primary: change in eGFR at 12 monthsSecondary: technical/procedural success, LVMI, BP, renal resistance index, kidney length, restenosis, QOL, NYHA classification, CV events and mortality, renal events and mortality, revascularization | Non-significant between-group differences in eGFR change at 12 months and the secondary endpointsAt 3 years, 29.4% from the medical group underwent revascularization (versus 3.0% in the PTRA group) |
DDD: daily defined dose; eCrCl: estimated creatinine clearance; FU: follow-up; LVMI: left ventricular mass index; NYHA: New York Heart Association; QOL: quality of life.
In the Dutch Renal Artery Stenosis (DRASTIC) study, 106 patients with ARVD (defined as lumen stenosis ≥50%) with normal or mildly impaired renal function were randomly assigned to PTRA or medical therapy [93]. No significant between-group differences in BP levels were detected at 3 and 12 months. However, almost half of the patients from the medical therapy group crossed over to the intervention group after 3 months due to uncontrolled BP despite treatment with three or more drugs or due to deterioration in renal function [2, 8]. In the Essai Multicentrique Medicaments vs Angioplastie (EMMA) [94] and the Scottish and Newcastle Renal Artery Stenosis Collaborative Group studies [95], no additional benefit from PTRA in addition to medical therapy in terms of BP control was shown, although in EMMA a lower number of antihypertensive medications was required in the active group and PTRA was associated with lower BP levels in patients with bilateral ARVD. These studies also had significant limitations, mainly relevant to a properly followed patient inclusion process. In EMMA, 27 of 76 originally eligible patients were excluded because of unexplained ‘physician refusal’ [94], while in the Scottish and Newcastle study, only 55 of the initial 135 eligible patients considered were finally randomized in the study protocol; the remaining subjects were not randomised because of unclarified ‘critical renal status’, ‘unstable BP’ or unexplained ‘multiple medical problems’ [95]. The Nephropathy Ischemic Therapy (NITER) trial [96] included patients with hypertension, GFR ≥30 ml/min and RAS ≥70%. It prematurely terminated with only 51 patients being enrolled (of 80 participants that were initially planned) due to slow enrolment and reported no primary results. A post hoc analysis [97] stratified patients into two groups according to median urinary albumin levels (≤0.04 g/24 h or >0.04 g/24 h) and showed no differences between PTRA with stenting and medical treatment alone in the composite of all-cause mortality, dialysis and CV events in the high albuminuria group, but event-free survival rates of 83% versus 45%, respectively, in the low albuminuria group.
A decade later, two multicentre studies compared PTRA with stenting plus medical therapy versus medical treatment alone for the prevention of kidney disease progression in patients with ARVD. The Stent Placement for Atherosclerotic Stenosis of Renal Artery (STAR) trial randomly assigned 140 patients with ARVD (defined as a reduction in the luminal diameter of the renal artery ≥50%), normal or impaired renal function (estimated creatinine clearance <80 but >15 ml/min/1.73 m2) and controlled BP while receiving a stable medication dosage in the month before inclusion [9]. The primary outcome was a 20% decrease in creatinine clearance during a 2-year follow-up. There was no difference between the two groups in the primary and several secondary endpoints, including changes in BP, incidence of refractory or malignant hypertension, pulmonary oedema, CV events and all-cause mortality. Again, however, almost one-third of the patients in the intervention group had a stenosis <50% at the time of angiography and were not eventually treated. Further, 30% of patients included in the medical therapy group and 44% in the stent group had arterial occlusion to a small or shrunken kidney‚ or both‚ although renal size <8 cm was an exclusion criterion [2, 9].
The Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) trial assigned 806 patients with evidence of renovascular disease and ‘substantial’ anatomical atherosclerotic stenosis (degree of stenosis not predefined) in at least one renal artery to revascularization plus medical therapy or to medical therapy alone [10]. The primary outcome was renal function, as measured by the reciprocal of the serum creatinine level (a measure that has a linear relationship with creatinine clearance). After 5 years of follow-up, there were no significant improvements in BP or reductions in the incidence of renal or CV events or mortality in the revascularization group and the benefits in terms of renal function were not clinically significant. However, the ASTRAL trial did not answer the question of intervention in severe ARVD. Patients were enrolled if there was a stenosis of >70% by non-invasive evaluation only ‘if the physician was uncertain if there would be benefit’ [10]. Thus patients considered likely to benefit from revascularization were excluded [2, 8]. In this context, <50% of the participants met current criteria for resistant hypertension. Moreover, 40% of the patients at angiography did not meet entry criteria for severe RAS. The average degree of stenosis was 76% (range 40–100) and 75% (range 20–99) in the revascularization and the medical treatment groups, respectively [10].
In the Renal Artery Stenosis in Coronary Artery Disease (RASCAD) trial [98], 84 patients undergoing cardiac catheterization for ischaemic heart disease and who had renal artery stenosis >50% but ≤80% were randomized to revascularization plus standard medical therapy versus medical therapy alone. After 1 year, there was no significant difference in the primary outcome between groups, i.e. change in echocardiographic left ventricular mass index. The study had no inclusion criterion relevant to hypertension or other ARVD phenotypes, had slow enrolment rates and a final population that was half of that planned.
The multicentre Cardiovascular Outcomes with Renal Atherosclerotic Lesions (CORAL) study [11] originally aimed to enrol 947 patients with ARVD and either uncontrolled systolic hypertension while taking two or more antihypertensive drugs or CKD and RAS >80% but <100% of the diameter or RAS >60% but <80% with a systolic pressure gradient >20 mmHg. The primary endpoint was a composite of death from CV or renal causes, myocardial infarction, stroke and hospitalization for CHF, progressive renal insufficiency or the need for renal replacement therapy. The CORAL study replicated the findings of the STAR and ASTRAL trials and also showed no additional benefit in the composite primary endpoint when compared with medical therapy alone. However, again, several methodological issues appeared. Similar to the other clinical trials, patients with a presentation suggestive of critical RAS (flash pulmonary oedema, refractory hypertension or rapid loss of kidney function after ACEI/ARB use) were excluded. Several protocol modifications to expand the inclusion criteria were made due to very slow enrolment. Uncontrolled hypertension was no longer required and >25% of patients were already at goal. Angiographically documented stenosis was no longer an inclusion criterion and in one out of four patients the diagnosis was based only on renal ultrasound. As such, although a stenosis >70% was an inclusion criterion, the CORAL cohort had a mean RAS of 67%, with <50% of patients having severe disease (>80% stenosis). Lastly, the more recently published RADAR trial [99], including 86 patients with ‘hemodynamically relevant’ RAS and a follow-up period of 3 years, showed no significant differences between PTRA with stenting plus best medical treatment versus medical treatment alone in all studied outcomes. However, this trial was also terminated due to very slow enrolment (86 of the 300 initially scheduled participants) and had high crossover rates (29.4% of patients in the medical group underwent revascularization).
Major limitations of RCTs in the field and evolution of treatment practices
The aforementioned RCTs attempted to properly examine whether revascularization of the renal artery is superior or not to medical treatment alone in ARVD. An objective reader should note that investigators of more recent trials tried to avoid the limitations of early RCTs; this is exemplified in the original design of the CORAL trial, which included a large population, a clinically relevant cardiorenal primary endpoint and required both resistant hypertension and angiographically documented severe RAS [11]. However, the complexity of the disease under study led to very slow recruitment and protocol amendments that introduced bias. Overall, a set of major limitations are common for these RCTs [2, 32, 100–102] (Table 3). All of them had non-standardized inclusion criteria, resulting in enrolment of large numbers of patients with mild/asymptomatic RAS, mild hypertension or advanced CKD with small kidneys, i.e. individuals with almost certain absence of benefit from RAS revascularization. The presence of systematic biases in radiological assessment of RAS and poor laboratory proof of critical RAS is also highly possible, as there was large variability not only between, but also within study protocols in imaging techniques used for RAS diagnosis and evaluation, often resulting in overestimation of the degree of stenosis. Additional methodological limitations include a large number of patients fulfilling the inclusion criteria that were not randomized based on investigators’ judgment without specific justification. Large enrolment delays were present for all major trials and led to protocol amendments during the trial that greatly changed the composition of the studied population. Further, high crossover rates between the study arms and low event rates for major outcomes were present in several cases [2, 32, 100–102].
Table 3:
Major limitations of RCTs in the field of ARVD.
| • Non-standardized inclusion criteria• Inclusion of patients with mild/asymptomatic RAS, mild hypertension or advanced CKD• Exclusion of patients with clinical presentation suggestive of critical RAS (recurrent flash pulmonary oedema, resistant hypertension, progressive renal function decline)• Great variability between and within study protocols in imaging techniques of RAS diagnosis and evaluation, often resulting in overestimation of the degree of stenosis• Enrolment delays• Protocol revisions during the trial• High crossover rates between treatment arms• Low event rates of major outcomes |
Overall, the diverse trials also demonstrated the difficulties encountered in performing trials in subjects with ARVD, even before accounting for additional issues such as the potential variability in the level of expertise of physicians performing the procedures. In this regard, the few additional clinical trials examining possible benefits of revascularization in the field of ARVD identified in a systematic search of databases of clinical trials (Supplementary Table 2) appear to have been terminated without publishing results. As such, no major input in the field from major trials can be anticipated in near future.
The most important problem arising from the aforementioned RCTs is a large misinterpretation of their results by the medical community. As discussed, they have almost entirely excluded patients with clinical presentations highly suggestive of functionally important RAS, such as those with flash pulmonary oedema, refractory hypertension or rapid loss of kidney function after use of an ACEI or ARB. Although that this was a conscious and ethical choice, on the basis that the investigators truly considered these individuals to have a clear indication for revascularization, the fact that their results cannot be extrapolated to these specific subsets of patients with high-risk phenotypes was not properly emphasized. Thus these publications rather gave rise to widespread doubt of the utility of PTRA and many selected patients that could have significantly benefited, were deprived of the procedure [2, 8, 82]. To this end, several guideline and consensus documents in recent years have provided detailed recommendations against this nihilistic view of PTRA, indicating that revascularization should be performed in specific patient groups [19, 74, 103–106], as discussed in detail below.
Clinical phenotypes of ARVD that may benefit from revascularization
Based on the totality of the available evidence, there are several clinical phenotypes of ARVD that may benefit from revascularization: resistant hypertension, HF, patients with rapid deterioration of renal function and kidney transplant recipients (KTRs).
Resistant hypertension
Resistant hypertension is associated with increased risk of CV events and mortality and is most often due to secondary types of hypertension [17, 40]. The prevalence of ARVD is estimated to be 14–24% in patients with severe or resistant hypertension [14–16], but no RCT in the field included solely patients with properly defined resistant hypertension. Multiple prospective and retrospective studies of patients with ARVD and resistant hypertension who underwent PTRA indicate that BP levels and antihypertensive pill burden were significantly reduced after revascularization [2]. In a retrospective uncontrolled single-centre study in 72 patients with ARVD and resistant hypertension, PTRA with or without stenting significantly decreased ambulatory BP levels by 14.0 ± 17.3/6.4 ± 8.7 mmHg and the number of antihypertensive drugs (from 4.0 ± 1.0 to 3.6 ± 1.4) [107]. In an international registry (2001–2009) including 265 consecutive patients with ARVD (≥50% de novo stenosis) and at least one of the following: mean SBP ≥160 mmHg on at least three anti-hypertensive medications including a diuretic, eGFR <60 ml/min/1.73 m2, or unexplained CHF or recurrent acute pulmonary oedema that was treated by PTRA with stenting [91], BP was reduced from 160/86 mmHg to 135/75 mmHg. Finally, in the aforementioned study of 467 patients from Ritchie et al. [32], revascularization was associated with improved survival in patients with the combination of rapidly declining renal function and refractory hypertension [HR for death 0.15 (95% CI 0.02–0.9), P = .04].
HF
ARVD is highly prevalent in HF, occurring in 54% of outpatient individuals with HF with reduced EF [108] and in 34% of patients >70 years of age with hospitalization for acute systolic HF [108]. Two small secondary analyses of ASTRAL showed no difference between groups in cardiac structure and function, assessed either by echocardiography or MRI [109, 110]. However, as discussed above, HF was underrepresented in the large RCTs [2] and no imaging RCTs including solely patients with HF and RAS are available. Several case reports reported significant improvements in cardiac morphology and function after renal artery revascularization in patients with severe bilateral renal artery stenosis presenting with flash pulmonary oedema [111, 112]. Another observational analysis from the UK in 611 patients with RAS >50% (of which 152 patients had coexisting HF without previous pulmonary oedema) showed a large difference in mortality risk between PTRA and standard medical therapy for those with HF [HR 0.6 (95% CI 0.3–0.9)] and non-significant reductions for those without [HR 0.8 (95% CI 0.5–1.1)] [113]. Furthermore, the HR for hospital admission for HF overall in revascularised patients was 0.2 (95% CI 0.0–1.1, P = .06). In 152 patients with HF but without previous acute pulmonary oedema, the HR for death after revascularization compared with medical therapy was 0.76 (95% CI 0.58–0.99) [114]. In another observational study in 163 patients, PTRA with stenting was associated with a significant decrease in the New York Heart Association functional class (1.9 ± 0.8 versus 2.6 ± 1.0; P < .04) and a 5-fold reduction in the number of hospitalizations compared with medical treatment [53]. These data suggest that revascularization of RAS in HF is associated with a substantial reduction in all-cause mortality and hospital admission and call for an RCT of renal artery revascularization versus medical therapy in patients with RAS and HF. Until such evidence is available, on the basis of existing observational data and expert opinion, recurrent hospitalizations for HF and/or pulmonary oedema with severe ARVD are considered indications for PTRA. Of note, all this published experience preceded the introduction of several newer agents that constitute the current standard of therapy for HF, such as sacubitril–valsartan or sodium–glucose cotransporter-2 inhibitors [115, 116].
Patients with rapid deterioration of kidney function
Patients with rapid deterioration of kidney function and ARVD may benefit from revascularization, as has been shown by several small studies, case series and case reports [100, 117–120]. In selected patients with advanced CKD or recent initiation of dialysis, revascularization can also be beneficial in stabilization or significant recovery of kidney function [100, 118, 120–122]. Of note, participants who presented improved kidney function after PTRA with stenting have a lower risk of death and multiple CV and renal complications, as recently demonstrated in a subgroup analysis of the CORAL trial [123]. Most reports of salvage of renal function apply to patients with renovascular disease affecting the total functional mass (e.g. a solitary functioning kidney or high-grade bilateral disease) [90, 124]. Some patients continue to have relative preservation of renal size despite high-grade vascular occlusion. Preservation of volume may be related to the presence of collateral vessels that develop to replace a minimum level of perfusion, but most commonly indicate a stenosis that has recently become severe and functionally critical. As such, the reversibility of damage is time dependent and, regarding the patients who were receiving dialysis, there is a consensus to avoid revascularization in those on dialysis for >3 months [19].
KTRs
The occurrence of transplant RAS ranges from 1 to 23% and is associated with poor graft and patient survival [125, 126]. Endovascular treatment of transplant RAS is effective. Long-term graft and patient survival after endovascular correction of transplant RAS were similar to those without transplant RAS and most patients avoided returning to dialysis [127]. In a study from 1999, the incidence of transplant RAS was 6.6%, the technical success of angioplasty was 92.3% and restenosis occurred in 23.1%; revascularization resulted in improved BP control and improved renal function. The 8-year patient (100% versus 98.6%, respectively) and graft (88.1% versus 88.9%, respectively) survival rates were similar in patients with and without transplant RAS [128]. In a recent single-centre study including 62 patients undergoing PTRA for transplant RAS, the patency rates were 85% at 1 year and allograft survival rates were 97% at 1 year, 89% at 5 years and 85% at 10 years [129]. In a recent study, 65 cases of RAS were identified from 1072 patients who underwent kidney transplantation. One-year clinical success according to renal outcome and BP reduction was 78.5% and 49.2%, respectively. Both renal outcome (79.4% versus 77.4%; P = .845) and BP reduction (40.6% versus 58.1%; P = .166) at 1 year were similar between the PTRA and PTRA with stenting groups. Event-free survival for composite of kidney transplant graft failure or transplant renal artery restenosis was significantly higher in PTRA with stenting at 1 year, but similar between groups at 10 years [130].
Antithrombotic therapy following PTRA
The type and duration of antithrombotic therapy after renal artery PTRA has not been studied in specific RCTs. In the aforementioned trials comparing PTRA with stenting to medical treatment, different types of antithrombotics were used in most (but not all) patients included in the intervention arm, such as low-dose aspirin monotherapy [9] or combinations of low-dose aspirin with clopidogrel or ticagrelor [11, 99]. One observational study reported a trend towards fewer secondary procedures for revascularization failure if the initial stenting was followed by dual antiplatelet therapy [131]. Of note, the various stent devices (bare metal, drug eluting etc.) require different types and duration of antiplatelet treatment and the majority of patients with ARVD have additional atherosclerotic lesions on other vascular beds (coronary, carotid or peripheral arteries) that could have been previously treated with intravascular or open surgical procedures and require antithrombotic therapy [104]. In the absence of trials directly assessing the effects of dual versus single antiplatelet therapy after renal PTRA with stenting, antithrombotic therapy should be chosen in collaboration of the interventionists and the treating nephrologists and cardiologists on an individualized basis, taking into account all the patient's comorbidities, as well as the thrombotic and bleeding risk. In everyday clinical practice, a combination of clopidogrel and low-dose aspirin is empirically prescribed in most centres, typically from 1 to 3 months, prolonged in some cases up to 1 year, after which single antiplatelet therapy is used [104].
Complications of PTRA
With technical improvements in recent decades, complication rates of PTRA have decreased and occur in ≈5% of patients [132]. Most complications are minor and related with the vascular access site, usually the femoral artery (i.e. haematoma). Catastrophic events such as atheroemboli, dissection, renal artery rupture and thrombosis are rare [132]. Atheroembolism represents a common complication of any patient with atherosclerotic disease and may occur spontaneously irrespective of PTRA [133]; however, any catheter manipulation during the procedure increases the relative risk. Some studies have demonstrated that atheroemboli can be prevented by using embolic prevention [134–138]. The only prospective and randomized trial testing embolic prevention device (EPD) efficacy showed no differences in kidney function between renal artery stenting alone, stenting with EPD and stenting with glycoprotein IIb/IIIa inhibitors [139]. Arterial dissection and arterial perforation or rupture are rare complications that usually are successfully treated with an additional stent or a covered stent graft, respectively, without requiring surgery [132]. Finally, contrast medium–induced nephropathy occurs in <5% of patients undergoing PTRA; regular prevention measures with normal saline hydration should be incorporated if the patient is not fluid overloaded [140–142].
Surgical revascularization
High-quality evidence on the use of surgical revascularization for ARVD is scarce, since all major RCTs examined the efficacy and safety of PTRA [19]. A previous meta-analysis included 47 studies comparing open surgical revascularization versus PTRA for ARVD and showed a higher rate of improvement in kidney function with surgery but a 3.1% higher perioperative mortality [143]. In agreement with interventions in other vascular beds, open surgical revascularization should be limited to patients with recurrent stenosis after percutaneous interventions (as discussed below), patients not suitable for PTRA as a result of complex anatomy [144] or patients undergoing scheduled open repair for other diseases, most commonly with an abdominal aortic aneurysm [145].
Follow-up after revascularization
The initial clinical response to PTRA should be evaluated within the first week after the intervention [146]. Antihypertensive medications, kidney function and/or cardiac symptoms should be reassessed at follow-up and screening for restenosis should be considered in case of unexplained BP increase, kidney function decline, episode of pulmonary oedema or other symptoms/signs suggestive of renovascular stenosis. Restenosis is one of the major complications that can occur at any time after PTRA. The incidence rates of restenosis are not clear and range from 6 to 60% [147]. Despite initial reports suggesting that in-stent stenosis occurs early after PTRA, studies with longer follow-up periods showed that rates of restenosis within 1 year and within 5 years were 20% and 32%, respectively [148]. A number of clinical (i.e. smoking, obesity, non-adherence to standard medical therapy, female sex), technical (i.e. stent type, mismatched stent and lesion, high residual stenosis) and vessel-related (i.e. vessels with a diameter <5 mm, long lesion/stent, fibrosis/plaque adjacent to stent) risk factors have been identified [147]. Surveillance with renal artery duplex ultrasound is suggested in all patients with renal artery stents to assess patency. The presence of risk factors for in-stent stenosis can guide the timing and frequency of screening. Previous ungraded recommendations suggest that all patients should undergo duplex ultrasonography soon as after PTRA is performed, to establish baseline parameters and patency; surveillance studies at 6 months and 1 year, then at least yearly thereafter, are suggested [147]. For patients in whom duplex ultrasonography does not provide accurate data regarding vessel patency, CTA or angiography could be considered when clinical questions on restenosis arise.
Clinical practice recommendations
As discussed above, in view of the results and the possible limitations of RCTs in the field, as well as several pieces of observational data showing that PTRA is associated with renal and CV benefits in patients presenting with high-risk ARVD phenotypes, a progressive shift in relevant recommendations from different bodies has occurred in recent years [8]. The present group of experts suggests a careful evaluation of the degree of the stenosis and the viability of the renal parenchyma of the stenotic kidney in candidates for revascularization, as described in Table 4. In addition, we suggest a personalized approach to select patients who will benefit from revascularization based on the strong and moderately strong indications described in Table 5. Additional parameters that should be taken into account to estimate overall renal and cardiovascular benefit should include the patient's age, duration of hypertension, presence of proteinuria and presence of comorbid conditions from other organs.
Table 4:
Evaluation of the possible viability of the kidney with ARVD following revascularization.
| Variable | Likely to benefit | Unlikely to benefit |
|---|---|---|
| RAS degree | >70% | <50% |
| Kidney length (cm) | >8 cma | <7 cm |
| Renal resistive index | <0.8 | >0.8 |
| Cortical thickness | Cortex distinct, e.g. >0.5 cm | Loss of corticomedullary differentiation, no cortex |
aThe suggested kidney length thresholds are relevant to individuals with average body habitus (i.e. body surface area ≈1.73 m2). For patients with very high or very low body mass, possibly consider the ratio of kidney length to the patient's body mass index or body surface area to approximate kidney size in relation to patient's body habitus.
Table 5:
Indications of PTRA in patients with ARVD.
| Strong indications |
| • High-grade (>70%) RAS in association with one of the following criteria:• Resistant hypertension• New-onset or recently uncontrolled hypertension• Acute pulmonary oedema or acute decompensated HF• Rapid decline of eGFR (bilateral stenosis or solitary kidney)• ACEI or ARB intolerance (≥30% eGFR reduction)• Renal replacement treatment (with possibly viable renal parenchyma) if stenosis detected <3 months after renal replacement treatment or if uncontrolled hypertension with multiple (five or more) antihypertensive agents• AKI due to acute renal artery occlusion or high-grade stenosis• Kidney transplant with RAS |
| Moderately strong indications |
| • High-grade (>70%) RAS in association with one of the following criteria:• Chronic HF• Asymptomatic but either bilateral or supplying a solitary kidney with viable renal parenchyma (non-atrophic kidney, distinct renal cortex) |
CONCLUSIONS AND FUTURE PERSPECTIVES
ARVD is a common clinical problem with clinical presentations relevant to many medical specialties and important prognostic associations. In contrast to previous RCTs, several pieces of observational data showed that PTRA in addition to medical therapy is associated with future renal and CV benefits in patients presenting with high-risk ARVD phenotypes. As such, based on the best available evidence, PTRA should be offered in selected individuals after careful evaluation. Future studies in the field should focus on several issues requiring further investigation that include but are not limited to those presented in Table 6.
Table 6:
Areas for further clinical research in the field of ARVD
| • RCTs enrolling patients with haemodynamically significant ARVD and high-risk clinical presentations, with true renovascular hypertension rather than patients with primary hypertension and incidental RAS through a wider and more systematic use of the translesional pressure gradient• Studies testing the impact of functional non-invasive imaging, such as BOLD MRI, to identify patients more likely to benefit from revascularization• Studies examining the efficacy of PTRA on moderate versus advanced CKD• Studies establishing the optimal timeline of revascularization to avoid delay-related ineffectiveness• Studies identifying predictors of PTRA benefit• Studies evaluating the efficacy of PTRA in combination with novel therapeutic strategies (e.g. targeting inflammation-related pathways, mesenchymal stem cells or angiogenic/growth factors) |
Supplementary Material
ACKNOWLEDGEMENTS
The ERBP is an official body of the European Renal Association.
Contributor Information
Pantelis A Sarafidis, Department of Nephrology, Hippokration Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Marieta Theodorakopoulou, Department of Nephrology, Hippokration Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece.
Alberto Ortiz, Department of Nephrology and Hypertension, IIS-Fundacion Jimenez Diaz UAM, Madrid, Spain.
Beatriz Fernandez-Fernández, Department of Nephrology and Hypertension, IIS-Fundacion Jimenez Diaz UAM, Madrid, Spain.
Ionut Nistor, Department of Internal Medicine, Nephrology and Geriatrics, Grigore T Popa University of Medicine and Pharmacy, Iasi, Romania; Department of Nephrology, Dr C I Parhon University Hospital, Iasi, Romania.
Roland Schmieder, Department of Nephrology and Hypertension, University Hospital Erlangen, Erlangen, Germany.
Mustafa Arici, Department of Nephrology, Hacettepe University Faculty of Medicine, Ankara, Turkey.
Athanasios Saratzis, Department of Cardiovascular Sciences & Leicester Vascular Institute, University Hospital Leicester, Leicester, UK.
Patricia Van der Niepen, Department of Nephrology & Hypertension, Universitair ziekenhuis Brussel, Vrije Universiteit Brussel, Brussels, Belgium.
Jean-Michel Halimi, Service de Néphrologie-Hypertension, Dialyses, Transplantation rénale, CHRU Tours, Tours, France and INSERM SPHERE U1246, Université Tours, Université de Nantes, Tours, France.
Reinhold Kreutz, Charité-Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Institut für Klinische Pharmakologie und Toxikologie, Berlin, Germany.
Andrzej Januszewicz, Department of Hypertension, National Institute of Cardiology, Warsaw, Poland.
Alexandre Persu, Division of Cardiology, Cliniques Universitaires Saint-Luc and Pole of Cardiovascular Research, Institut de Recherche Expérimentale et Clinique, Université Catholique de Louvain, Brussels, Belgium.
Mario Cozzolino, Renal Division, ASST Santi Paolo e Carlo, Department of Health Sciences, University of Milan, Milan, Italy.
FUNDING
A.O. and B.F.F. are supported by FIS/Fondos FEDER (PI20/00744, PI22/00050, Instituto de Salud Carlos III (ISCIII) RICORS program to RICORS2040 (RD21/0005/0001) funded by European Union–NextGenerationEU, Mecanismo para la Recuperación y la Resiliencia (MRR).
CONFLICT OF INTEREST STATEMENT
B.F.F. has received grants from Esteve and Mundipharma and consultancy or speaker fees or travel support from Springer, AstraZeneca, Bayer, Menarini, Novo Nordisk, Boehringer Ingelheim and Mundipharma and is Editor for Nefrologia Editorial Board (Nefroplus). M.A. has received payment or honoraria for lectures, presentations, speakers’ bureaus, publication writing or educational events from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, MSD, Novo Nordisk, Sandoz and Sanofi. A.S. has received honoraria, lecture fees, research funding, training fees and consultancy fees from Abbott, Shockwave, Amgen and Boston Scientific and proctoring fees from Abbott and Bentley. The other authors disclose that they do not have any financial or other relationships that might lead to a conflict of interest regarding this article.
REFERENCES
- 1. Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med 2001;344:431–42. 10.1056/NEJM200102083440607 [DOI] [PubMed] [Google Scholar]
- 2. Van der Niepen P, Rossignol P, Lengelé J-P et al. Renal artery stenosis in patients with resistant hypertension: stent it or not? Curr Hypertens Rep 2017;19:5. 10.1007/s11906-017-0703-8 [DOI] [PubMed] [Google Scholar]
- 3. Gornik HL, Persu A, Adlam D et al. First international consensus on the diagnosis and management of fibromuscular dysplasia. J Hypertens 2019;37:229–52. 10.1097/HJH.0000000000002019 [DOI] [PubMed] [Google Scholar]
- 4. Persu A, Canning C, Prejbisz A et al. Beyond atherosclerosis and fibromuscular dysplasia: rare causes of renovascular hypertension. Hypertension 2021;78:898–911. 10.1161/HYPERTENSIONAHA.121.17004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prince M, Tafur JD, White CJ. When and how should we revascularize patients with atherosclerotic renal artery stenosis? JACC Cardiovasc Interv 2019;12:505–17. 10.1016/j.jcin.2018.10.023 [DOI] [PubMed] [Google Scholar]
- 6. Goldblatt H, Lynch J, Hanzal RF et al. Studies on experimental hypertension: I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med 1934;59:347–79. 10.1084/jem.59.3.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goldblatt H. Renovascular hypertension due to renal ischemia. Circulation 1965;32:1–4. 10.1161/01.CIR.32.1.1 [DOI] [PubMed] [Google Scholar]
- 8. Theodorakopoulou MP, Karagiannidis AG, Ferro CJ et al. Renal artery stenting in the correct patients with atherosclerotic renovascular disease: time for a proper renal and cardiovascular outcome study? Clin Kidney J 2023;16:201–4. 10.1093/ckj/sfac140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bax L, Woittiez A-JJ, Kouwenberg HJ et al. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function. Ann Intern Med 2009;150:840–8. 10.7326/0003-4819-150-12-200906160-00119 [DOI] [PubMed] [Google Scholar]
- 10. Investigators ASTRAL, Wheatley K, Ives N et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009;361:1953–62. [DOI] [PubMed] [Google Scholar]
- 11. Cooper CJ, Murphy TP, Cutlip DE et al. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 2014;370:13–22. 10.1056/NEJMoa1310753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hansen KJ, Edwards MS, Craven TE et al. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg 2002;36:443–51. 10.1067/mva.2002.127351 [DOI] [PubMed] [Google Scholar]
- 13. Berglund G, Andersson O, Wilhelmsen L. Prevalence of primary and secondary hypertension: studies in a random population sample. BMJ 1976;2:554–6. 10.1136/bmj.2.6035.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khosla S, Kunjummen B, Manda R et al. Prevalence of renal artery stenosis requiring revascularization in patients initially referred for coronary angiography. Catheter Cardiovasc Interv 2003;58:400–3. 10.1002/ccd.10387 [DOI] [PubMed] [Google Scholar]
- 15. Buller CE, Nogareda JG, Ramanathan K et al. The profile of cardiac patients with renal artery stenosis. J Am Coll Cardiol 2004;43:1606–13. 10.1016/j.jacc.2003.11.050 [DOI] [PubMed] [Google Scholar]
- 16. Benjamin MM, Fazel P, Filardo G et al. Prevalence of and risk factors of renal artery stenosis in patients with resistant hypertension. Am J Cardiol 2014;113:687–90. 10.1016/j.amjcard.2013.10.046 [DOI] [PubMed] [Google Scholar]
- 17. Williams B, Mancia G, Spiering W et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens 2018;36:1953–2041. [DOI] [PubMed] [Google Scholar]
- 18. de Mast Q, Beutler JJ. The prevalence of atherosclerotic renal artery stenosis in risk groups: a systematic literature review. J Hypertens 2009;27:1333–40. 10.1097/HJH.0b013e328329bbf4 [DOI] [PubMed] [Google Scholar]
- 19. Hicks CW, Clark TWI, Cooper CJ et al. Atherosclerotic renovascular disease: a KDIGO (Kidney Disease: Improving Global Outcomes) controversies conference. Am J Kidney Dis 2022;79:289–301. 10.1053/j.ajkd.2021.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Studzińska D, Rudel B, Polok K et al. Infrarenal versus suprarenal abdominal aortic aneurysms: comparison of associated aneurysms and renal artery stenosis. Ann Vasc Surg 2019;58:248–254.e1. 10.1016/j.avsg.2018.10.044 [DOI] [PubMed] [Google Scholar]
- 21. Sarafidis P, Martens S, Saratzis A et al. Diseases of the aorta and kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Cardiovasc Res 2022;118:2582–95. 10.1093/cvr/cvab287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Silva R, Loh H, Rigby AS et al. Epidemiology, associated factors, and prognostic outcomes of renal artery stenosis in chronic heart failure assessed by magnetic resonance angiography. Am J Cardiol 2007;100:273–9. 10.1016/j.amjcard.2007.02.098 [DOI] [PubMed] [Google Scholar]
- 23. Jacobson HR. Ischemic renal disease: an overlooked clinical entity? Kidney Int 1988;34:729–43. 10.1038/ki.1988.240 [DOI] [PubMed] [Google Scholar]
- 24. Appel RG, Bleyer AJ, Reavis S et al. Renovascular disease in older patients beginning renal replacement therapy. Kidney Int 1995;48:171–6. 10.1038/ki.1995.281 [DOI] [PubMed] [Google Scholar]
- 25. Baboolal K, Evans C, Moore R. Incidence of end-stage renal disease in medically treated patients with severe bilateral atherosclerotic renovascular disease. Am J Kidney Dis 1998;31:971–7. 10.1053/ajkd.1998.v31.pm9631841 [DOI] [PubMed] [Google Scholar]
- 26. Leertouwer TC, Pattynama PMT, Van Den Berg-Huysmans A. Incidental renal artery stenosis in peripheral vascular disease: a case for treatment? Kidney Int 2001;59:1480–3. 10.1046/j.1523-1755.2001.0590041480.x [DOI] [PubMed] [Google Scholar]
- 27. Mailloux LU, Napolitano B, Bellucci AG et al. Renal vascular disease causing end-stage renal disease, incidence, clinical correlates, and outcomes: a 20-year clinical experience. Am J Kidney Dis 1994;24:622–9. 10.1016/S0272-6386(12)80223-X [DOI] [PubMed] [Google Scholar]
- 28. van Ampting JMA, Penne EL, Beek FJA et al. Prevalence of atherosclerotic renal artery stenosis in patients starting dialysis. Nephrol Dial Transplant 2003;18:1147–51. 10.1093/ndt/gfg121 [DOI] [PubMed] [Google Scholar]
- 29. Guo H, Kalra PA, Gilbertson DT et al. Atherosclerotic renovascular disease in older US patients starting dialysis, 1996 to 2001. Circulation 2007;115:50–8. 10.1161/CIRCULATIONAHA.106.637751 [DOI] [PubMed] [Google Scholar]
- 30. Kalra PA, Guo H, Kausz AT et al. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int 2005;68:293–301. 10.1111/j.1523-1755.2005.00406.x [DOI] [PubMed] [Google Scholar]
- 31. Ritchie J, Green D, Alderson HV et al. Risks for mortality and renal replacement therapy in atherosclerotic renovascular disease compared with other causes of chronic kidney disease. Nephrology 2015;20:688–96. 10.1111/nep.12501 [DOI] [PubMed] [Google Scholar]
- 32. Ritchie J, Green D, Chrysochou C et al. High-risk clinical presentations in atherosclerotic renovascular disease: prognosis and response to renal artery revascularization. Am J Kidney Dis 2014;63:186–97. 10.1053/j.ajkd.2013.07.020 [DOI] [PubMed] [Google Scholar]
- 33. Textor SC, Lerman LO. Paradigm shifts in atherosclerotic renovascular disease: where are we now? J Am Soc Nephrol 2015;26:2074–80. 10.1681/ASN.2014121274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hill GS, Heudes D, Bariéty J. Morphometric study of arterioles and glomeruli in the aging kidney suggests focal loss of autoregulation. Kidney Int 2003;63:1027–36. 10.1046/j.1523-1755.2003.00831.x [DOI] [PubMed] [Google Scholar]
- 35. Christensen PK, Hansen HP, Parving HH. Impaired autoregulation of GFR in hypertensive non-insulin dependent diabetic patients. Kidney Int 1997;52:1369–74. 10.1038/ki.1997.463 [DOI] [PubMed] [Google Scholar]
- 36. Lee SM, Drach GW. Renovascular hypertension from segmental renal artery stenosis: importance of segmental renal vein renin sampling. J Urol 1980;124:704–6. 10.1016/S0022-5347(17)55618-9 [DOI] [PubMed] [Google Scholar]
- 37. Aoi W, Akahoshi M, Seto S et al. Correction of hypertension by partial nephrectomy in segmental renal artery stenosis and electron microscopic studies of renin. Jpn Heart J 1981;22:679–87. 10.1536/ihj.22.679 [DOI] [PubMed] [Google Scholar]
- 38. Sarafidis PA, Georgianos PI, Germanidis G et al. Hypertension and symptomatic hypokalemia in a patient with simultaneous unilateral stenoses of intrarenal arteries and mesangioproliferative glomerulonephritis. Am J Kidney Dis 2012;59:434–8. 10.1053/j.ajkd.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 39. Kawarada O, Yasuda S, Noguchi T et al. Renovascular heart failure: heart failure in patients with atherosclerotic renal artery disease. Cardiovasc Interv Ther 2016;31:171–82. 10.1007/s12928-016-0392-2 [DOI] [PubMed] [Google Scholar]
- 40. Januszewicz A, Mulatero P, Dobrowolski P et al. Cardiac phenotypes in secondary hypertension: JACC state-of-the-art review. J Am Coll Cardiol 2022;80:1480–97. 10.1016/j.jacc.2022.08.714 [DOI] [PubMed] [Google Scholar]
- 41. Brilla CG. Renin-angiotensin-aldosterone system and myocardial fibrosis. Cardiovasc Res 2000;47:1–3. 10.1016/S0008-6363(00)00092-4 [DOI] [PubMed] [Google Scholar]
- 42. Díaz HS, Toledo C, Andrade DC et al. Neuroinflammation in heart failure: new insights for an old disease. J Physiol 2020;598:33–59. 10.1113/JP278864 [DOI] [PubMed] [Google Scholar]
- 43. Alexandrou M-E, Theodorakopoulou MP, Kanbay M et al. Mineralocorticoid receptor antagonists for cardioprotection in chronic kidney disease: a step into the future. J Hum Hypertens 2022;36:695–704. 10.1038/s41371-021-00641-1 [DOI] [PubMed] [Google Scholar]
- 44. Pathak AS, Huang J, Rojas M et al. Effects of restoration of blood flow on the development of aortic atherosclerosis in ApoE-/- mice with unilateral renal artery stenosis. J Am Heart Assoc 2016;5:e002953. 10.1161/JAHA.115.002953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stouffer GA, Pathak A, Rojas M. Unilateral renal artery stenosis causes a chronic vascular inflammatory response in ApoE−/− mice. Trans Am Clin Climatol Assoc 2010;121:252–66. [PMC free article] [PubMed] [Google Scholar]
- 46. Kashyap S, Warner G, Hu Z et al. Cardiovascular phenotype in Smad3 deficient mice with renovascular hypertension. PLoS One 2017;12:e0187062. 10.1371/journal.pone.0187062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nargesi AA, Farah MC, Zhu X-Y et al. Renovascular hypertension induces myocardial mitochondrial damage, contributing to cardiac injury and dysfunction in pigs with metabolic syndrome. Am J Hypertens 2021;34:172–82. 10.1093/ajh/hpaa202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Farahani RA, Yu S, Ferguson CM et al. Renal revascularization attenuates myocardial mitochondrial damage and improves diastolic function in pigs with metabolic syndrome and renovascular hypertension. J Cardiovasc Transl Res 2022;15:15–26. 10.1007/s12265-021-10155-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sarafidis PA, Georgianos P, Bakris GL. Resistant hypertension—its identification and epidemiology. Nat Rev Nephrol 2013;9:51–8. 10.1038/nrneph.2012.260 [DOI] [PubMed] [Google Scholar]
- 50. Lazaridis AA, Sarafidis PA, Ruilope LM. Ambulatory blood pressure monitoring in the diagnosis, prognosis, and management of resistant hypertension: still a matter of our resistance? Curr Hypertens Rep 2015;17:78. 10.1007/s11906-015-0590-9 [DOI] [PubMed] [Google Scholar]
- 51. Rimmer JM, Gennari FJ. Atherosclerotic renovascular disease and progressive renal failure. Ann Intern Med 1993;118:712–9. 10.7326/0003-4819-118-9-199305010-00010 [DOI] [PubMed] [Google Scholar]
- 52. Messerli FH, Bangalore S, Makani H et al. Flash pulmonary oedema and bilateral renal artery stenosis: the Pickering syndrome. Eur Heart J 2011;32:2231–5. 10.1093/eurheartj/ehr056 [DOI] [PubMed] [Google Scholar]
- 53. Kane GC, Xu N, Mistrik E et al. Renal artery revascularization improves heart failure control in patients with atherosclerotic renal artery stenosis. Nephrol Dial Transplant 2010;25:813–20. 10.1093/ndt/gfp393 [DOI] [PubMed] [Google Scholar]
- 54. Halimi JM, Ribstein J, Du Cailar G et al. Nephrotic-range proteinuria in patients with renovascular disease. Am J Med 2000;108:120–6. 10.1016/S0002-9343(99)00411-8 [DOI] [PubMed] [Google Scholar]
- 55. Eiser AR, Katz SM, Swartz C. Reversible nephrotic range proteinuria with renal artery stenosis: a clinical example of renin-associated proteinuria. Nephron 1982;30:374–7. 10.1159/000182521 [DOI] [PubMed] [Google Scholar]
- 56. Ben-Chitrit S, Korzets Z, Podjarny E et al. Reversal of the nephrotic syndrome due to renovascular hypertension by successful percutaneous angioplasty and stenting. Nephrol Dial Transplant 1995;10:1460–1. [PubMed] [Google Scholar]
- 57. Ubara Y, Hara S, Katori H et al. Renovascular hypertension may cause nephrotic range proteinuria and focal glomerulosclerosis in contralateral kidney. Clin Nephrol 1997;48:220–3. [PubMed] [Google Scholar]
- 58. Gephardt GN, Tubbs RR, Novick AC et al. Renal artery stenosis, nephrotic-range proteinuria, and focal and segmental glomerulosclerosis. Cleve Clin J Med 1984;51:371–6. 10.3949/ccjm.51.2.371 [DOI] [PubMed] [Google Scholar]
- 59. Halimi JM, Ribstein J, Du Cailar G et al. Albuminuria predicts renal functional outcome after intervention in atheromatous renovascular disease. J Hypertens 1995;13:1335–42. 10.1097/00004872-199511000-00016 [DOI] [PubMed] [Google Scholar]
- 60. Williams GJ, Macaskill P, Chan SF et al. Comparative accuracy of renal duplex sonographic parameters in the diagnosis of renal artery stenosis: paired and unpaired analysis. Am J Roentgenol 2007;188:798–811. 10.2214/AJR.06.0355 [DOI] [PubMed] [Google Scholar]
- 61. Davies MG, Saad WE, Bismuth J et al. Renal parenchymal preservation after percutaneous renal angioplasty and stenting. J Vasc Surg 2010;51:1222–9. 10.1016/j.jvs.2009.09.050 [DOI] [PubMed] [Google Scholar]
- 62. Soulez G, Therasse E, Qanadli SD et al. Prediction of clinical response after renal angioplasty: respective value of renal doppler sonography and scintigraphy. Am J Roentgenol 2003;181:1029–35. 10.2214/ajr.181.4.1811029 [DOI] [PubMed] [Google Scholar]
- 63. Soares GM, Murphy TP, Singha MS et al. Renal artery duplex ultrasonography as a screening and surveillance tool to detect Renal artery stenosis. J Ultrasound Med 2006;25:293–8. 10.7863/jum.2006.25.3.293 [DOI] [PubMed] [Google Scholar]
- 64. van Jaarsveld BC, Krijnen P, Derkx FH et al. The place of renal scintigraphy in the diagnosis of renal artery stenosis. Fifteen years of clinical experience. Arch Intern Med 1997;157:1226–34. 10.1001/archinte.1997.00440320128012 [DOI] [PubMed] [Google Scholar]
- 65. Vasbinder GBC, Nelemans PJ, Kessels AGH et al. Accuracy of computed tomographic angiography and magnetic resonance angiography for diagnosing renal artery stenosis. Ann Intern Med 2004;141:674–82. 10.7326/0003-4819-141-9-200411020-00007 [DOI] [PubMed] [Google Scholar]
- 66. Davenport MS, Khalatbari S, Dillman JR et al. Contrast material–induced nephrotoxicity and intravenous low-osmolality iodinated contrast material. Radiology 2013;267:94–105. 10.1148/radiol.12121394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. McDonald JS, McDonald RJ, Carter RE et al. Risk of intravenous contrast material–mediated acute kidney injury: a propensity score–matched study stratified by baseline-estimated glomerular filtration rate. Radiology 2014;271:65–73. 10.1148/radiol.13130775 [DOI] [PubMed] [Google Scholar]
- 68. Woolen SA, Shankar PR, Gagnier JJ et al. Risk of nephrogenic systemic fibrosis in patients with stage 4 or 5 chronic kidney disease receiving a group II gadolinium-based contrast agent: a systematic review and meta-analysis. JAMA Intern Med 2020;180:223–30. 10.1001/jamainternmed.2019.5284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chrysochou C, Mendichovszky IA, Buckley DL et al. BOLD imaging: a potential predictive biomarker of renal functional outcome following revascularization in atheromatous renovascular disease. Nephrol Dial Transplant 2012;27:1013–9. 10.1093/ndt/gfr392 [DOI] [PubMed] [Google Scholar]
- 70. Gloviczki ML, Saad A, Textor SC. Blood oxygen level-dependent (BOLD) MRI analysis in atherosclerotic renal artery stenosis. Curr Opin Nephrol Hypertens 2013;22:519–24. 10.1097/MNH.0b013e32836400b2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pruijm M, Mendichovszky IA, Liss P et al. Renal blood oxygenation level-dependent magnetic resonance imaging to measure renal tissue oxygenation: a statement paper and systematic review. Nephrol Dial Transplant 2018;33:ii22–8. 10.1093/ndt/gfy243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. May AG, De Weese JA, Rob CG. Hemodynamic effects of arterial stenosis. Surgery 1963;53:513–24. [PubMed] [Google Scholar]
- 73. Imanishi M, Akabane S, Takamiya M et al. Critical degree of renal arterial stenosis that causes hypertension in dogs. Angiology 1992;43:833–42. 10.1177/000331979204301006 [DOI] [PubMed] [Google Scholar]
- 74. Klein AJ, Jaff MR, Gray BH et al. SCAI appropriate use criteria for peripheral arterial interventions: an update. Catheter Cardiovasc Interv 2017;90:E90–110. 10.1002/ccd.27141 [DOI] [PubMed] [Google Scholar]
- 75. Losito A, Gaburri M, Errico R et al. Survival of patients with renovascular disease and ACE inhibition. Clin Nephrol 1999;52:339–43. [PubMed] [Google Scholar]
- 76. Hackam DG, Duong-Hua ML, Mamdani M et al. Angiotensin inhibition in renovascular disease: a population-based cohort study. Am Heart J 2008;156:549–55. 10.1016/j.ahj.2008.05.013 [DOI] [PubMed] [Google Scholar]
- 77. Chrysochou C, Foley RN, Young JF et al. Dispelling the myth: the use of renin–angiotensin blockade in atheromatous renovascular disease. Nephrol Dial Transplant 2012;27:1403–9. 10.1093/ndt/gfr496 [DOI] [PubMed] [Google Scholar]
- 78. Sun D, Chen Z, Eirin A et al. Hypercholesterolemia impairs nonstenotic kidney outcomes after reversal of experimental renovascular hypertension. Am J Hypertens 2016;29:853–9. 10.1093/ajh/hpv222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Visseren FLJ, Mach F, Smulders YM et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021;42:3227–337. 10.1093/eurheartj/ehab484 [DOI] [PubMed] [Google Scholar]
- 80. Ritchie J, Green D, Alderson HV et al. Associations of antiplatelet therapy and beta blockade with patient outcomes in atherosclerotic renovascular disease. J Am Soc Hypertens 2016;10:149–158.e3. 10.1016/j.jash.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 81. Bhalla V, Textor SC, Beckman JA et al. Revascularization for renovascular disease: a scientific statement from the American Heart Association. Hypertension 2022;79:e128–43. 10.1161/HYP.0000000000000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pappaccogli M, Robberechts T, Lengelé J-P et al. Endovascular versus medical management of atherosclerotic renovascular disease: update and emerging concepts. Hypertension 2023;80:1150–61. 10.1161/HYPERTENSIONAHA.122.17965. [DOI] [PubMed] [Google Scholar]
- 83. Steinbach F, Novick AC, Campbell S et al. Long-term survival after surgical revascularization for atherosclerotic renal artery disease. J Urol 1997;158:38–41. 10.1097/00005392-199707000-00011 [DOI] [PubMed] [Google Scholar]
- 84. Hansen KJ, Cherr GS, Craven TE et al. Management of ischemic nephropathy: dialysis-free survival after surgical repair. J Vasc Surg 2000;32:472–82. 10.1067/mva.2000.108637 [DOI] [PubMed] [Google Scholar]
- 85. Cambria RP, Brewster DC, L'Italien GJ et al. Renal artery reconstruction for the preservation of renal function. J Vasc Surg 1996;24:371–380; discussion 371–82. 10.1016/S0741-5214(96)70193-3 [DOI] [PubMed] [Google Scholar]
- 86. Weibull H, Bergqvist D, Bergentz SE et al. Percutaneous transluminal renal angioplasty versus surgical reconstruction of atherosclerotic renal artery stenosis: a prospective randomized study. J Vasc Surg 1993;18:841–50; discussion 850–852. 10.1016/0741-5214(93)90340-R [DOI] [PubMed] [Google Scholar]
- 87. Burket MW, Cooper CJ, Kennedy DJ et al. Renal artery angioplasty and stent placement: predictors of a favorable outcome. Am Heart J 2000;139:64–71. 10.1016/S0002-8703(00)90310-7 [DOI] [PubMed] [Google Scholar]
- 88. Harden PN, MacLeod MJ, Rodger RS et al. Effect of renal-artery stenting on progression of renovascular renal failure. Lancet 1997;349:1133–6. 10.1016/S0140-6736(96)10093-3 [DOI] [PubMed] [Google Scholar]
- 89. Blum U, Krumme B, Flügel P et al. Treatment of ostial renal-artery stenoses with vascular endoprostheses after unsuccessful balloon angioplasty. N Engl J Med 1997;336:459–65. 10.1056/NEJM199702133360702 [DOI] [PubMed] [Google Scholar]
- 90. Watson PS, Hadjipetrou P, Cox SV et al. Effect of renal artery stenting on renal function and size in patients with atherosclerotic renovascular disease. Circulation 2000;102:1671–7. 10.1161/01.CIR.102.14.1671 [DOI] [PubMed] [Google Scholar]
- 91. Milewski K, Fil W, Buszman P et al. Renal artery stenting associated with improvement in renal function and blood pressure control in long-term follow-up. Kidney Blood Press Res 2016;41:278–87. 10.1159/000443423 [DOI] [PubMed] [Google Scholar]
- 92. Reinhard M, Schousboe K, Andersen UB et al. Renal artery stenting in consecutive high-risk patients with atherosclerotic renovascular disease: a prospective 2-center cohort study. J Am Heart Assoc 2022;11:e024421. 10.1161/JAHA.121.024421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. van Jaarsveld BC, Krijnen P, Pieterman H et al. The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group. N Engl J Med 2000;342:1007–14. 10.1056/NEJM200004063421403 [DOI] [PubMed] [Google Scholar]
- 94. Plouin PF, Chatellier G, Darné B et al. Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: a randomized trial. Essai Multicentrique Medicaments vs Angioplastie (EMMA) Study Group. Hypertension 1998;31:823–9. 10.1161/01.HYP.31.3.823 [DOI] [PubMed] [Google Scholar]
- 95. Webster J, Marshall F, Abdalla M et al. Randomised comparison of percutaneous angioplasty vs continued medical therapy for hypertensive patients with atheromatous renal artery stenosis. Scottish and Newcastle Renal Artery Stenosis Collaborative Group. J Hum Hypertens 1998;12:329–35. 10.1038/sj.jhh.1000599 [DOI] [PubMed] [Google Scholar]
- 96. Scarpioni R, Michieletti E, Cristinelli L et al. Atherosclerotic renovascular disease: medical therapy versus medical therapy plus renal artery stenting in preventing renal failure progression: the rationale and study design of a prospective, multicenter and randomized trial (NITER). J Nephrol 2005;18:423–8. [PubMed] [Google Scholar]
- 97. Siddiqui EU, Murphy TP, Naeem SS et al. Interaction between albuminuria and treatment group outcomes for patients with renal artery stenosis: the NITER study. J Vasc Interv Radiol 2018;29:966–70. 10.1016/j.jvir.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 98. Marcantoni C, Zanoli L, Rastelli S et al. Effect of renal artery stenting on left ventricular mass: a randomized clinical trial. Am J Kidney Dis 2012;60:39–46. 10.1053/j.ajkd.2012.01.022 [DOI] [PubMed] [Google Scholar]
- 99. Zeller T, Krankenberg H, Erglis A et al. A randomized, multi-center, prospective study comparing best medical treatment versus best medical treatment plus renal artery stenting in patients with hemodynamically relevant atherosclerotic renal artery stenosis (RADAR) – one-year results of a pre-maturely terminated study. Trials 2017;18:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sarafidis PA, Stavridis KC, Loutradis CN et al. To intervene or not? A man with multidrug-resistant hypertension, endovascular abdominal aneurysm repair, bilateral renal artery stenosis and end-stage renal disease salvaged with renal artery stenting. Blood Press 2016;25:123–8. 10.3109/08037051.2015.1110926 [DOI] [PubMed] [Google Scholar]
- 101. Messina LM, Zelenock GB, Yao KA et al. Renal revascularization for recurrent pulmonary edema in patients with poorly controlled hypertension and renal insufficiency: a distinct subgroup of patients with arteriosclerotic renal artery occlusive disease. J Vasc Surg 1992;15:73–80; discussion 80–82. 10.1016/0741-5214(92)70015-D [DOI] [PubMed] [Google Scholar]
- 102. Murphy TP, Cooper CJ, Cutlip DE et al. Roll-in experience from the Cardiovascular Outcomes with Renal Atherosclerotic Lesions (CORAL) study. J Vasc Interv Radiol 2014;25:511–20. 10.1016/j.jvir.2013.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Whelton PK, Carey RM, Aronow WS et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines. Hypertension 2018;71:e13–115. [DOI] [PubMed] [Google Scholar]
- 104. Aboyans V, Ricco J-B, Bartelink M-LEL et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries Endorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018;39:763–816. [DOI] [PubMed] [Google Scholar]
- 105. Umemura S, Arima H, Arima S et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res 2019;42:1235–481. 10.1038/s41440-019-0284-9 [DOI] [PubMed] [Google Scholar]
- 106. Johansen KL, Garimella PS, Hicks CW et al. Central and peripheral arterial diseases in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int 2021;100:35–48. 10.1016/j.kint.2021.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Courand P-Y, Dinic M, Lorthioir A et al. Resistant hypertension and atherosclerotic renal artery stenosis: effects of angioplasty on ambulatory blood pressure. A retrospective uncontrolled single-center study. Hypertension 2019;74:1516–23. 10.1161/HYPERTENSIONAHA.119.13393 [DOI] [PubMed] [Google Scholar]
- 108. MacDowall P, Kalra P, O'Donoghue D et al. Risk of morbidity from renovascular disease in elderly patients with congestive cardiac failure. Lancet 1998;352:13–6. 10.1016/S0140-6736(97)11060-1 [DOI] [PubMed] [Google Scholar]
- 109. Ritchie J, Green D, Chrysochou T et al. Effect of renal artery revascularization upon cardiac structure and function in atherosclerotic renal artery stenosis: cardiac magnetic resonance sub-study of the ASTRAL trial. Nephrol Dial Transplant 2017;32:1006–13. [DOI] [PubMed] [Google Scholar]
- 110. Green D, Vassallo D, Handley K et al. Cardiac structure and function after revascularization versus medical therapy for renal artery stenosis: the ASTRAL heart echocardiographic sub-study. BMC Nephrol 2019;20:220. 10.1186/s12882-019-1406-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chrysochou C, Schmitt M, Siddals K et al. Reverse cardiac remodelling and renal functional improvement following bilateral renal artery stenting for flash pulmonary oedema. Nephrol Dial Transplant 2013;28:479–83. 10.1093/ndt/gfr745 [DOI] [PubMed] [Google Scholar]
- 112. Bloch MJ, Trost DW, Pickering TG et al. Prevention of recurrent pulmonary edema in patients with bilateral renovascular disease through renal artery stent placement. Am J Hypertens 1999;12:1–7. 10.1016/S0895-7061(98)00201-5 [DOI] [PubMed] [Google Scholar]
- 113. Green D, Ritchie JP, Chrysochou C et al. Revascularisation of renal artery stenosis as a therapy for heart failure: an observational cohort study. Lancet 2015;385(Suppl 1):S11. 10.1016/S0140-6736(15)60326-9 [DOI] [PubMed] [Google Scholar]
- 114. Green D, Ritchie JP, Chrysochou C et al. Revascularization of atherosclerotic renal artery stenosis for chronic heart failure versus acute pulmonary oedema. Nephrology 2018;23:411–7. 10.1111/nep.13038 [DOI] [PubMed] [Google Scholar]
- 115. Heidenreich PA, Bozkurt B, Aguilar D et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: Executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022;79:1757–80. 10.1016/j.jacc.2021.12.011 [DOI] [PubMed] [Google Scholar]
- 116. McDonagh TA, Metra M, Adamo M et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2022;24:4–131. [DOI] [PubMed] [Google Scholar]
- 117. Thatipelli M, Misra S, Johnson CM et al. Renal artery stent placement for restoration of renal function in hemodialysis recipients with renal artery stenosis. J Vasc Interv Radiol 2008;19:1563–8. 10.1016/j.jvir.2008.08.016 [DOI] [PubMed] [Google Scholar]
- 118. de Bhailis Á, Al-Chalabi S, Hagemann R et al. Managing acute presentations of atheromatous renal artery stenosis. BMC Nephrol 2022;23:210. 10.1186/s12882-022-02813-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ramos F, Kotliar C, Alvarez D et al. Renal function and outcome of PTRA and stenting for atherosclerotic renal artery stenosis. Kidney Int 2003;63:276–82. 10.1046/j.1523-1755.2003.00734.x [DOI] [PubMed] [Google Scholar]
- 120. Takahashi W, Morita T, Tanaka K et al. Determinant role of renal artery stenting in recovery from acute worsening of atherosclerotic renal failure. J Cardiol Cases 2021;24:49–51. 10.1016/j.jccase.2020.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Kuznetsov E, Schifferdecker B, Jaber BL et al. Recovery of acute renal failure following bilateral renal artery angioplasty and stenting. Clin Nephrol 2007;68:32–7. 10.5414/CNP68032 [DOI] [PubMed] [Google Scholar]
- 122. Kanamori H, Toma M, Fukatsu A. Improvement of renal function after opening occluded atherosclerotic renal arteries. J Invasive Cardiol 2009;21:E171–4. [PubMed] [Google Scholar]
- 123. Modrall JG, Zhu H, Prasad T et al. Retrieval of renal function after renal artery stenting improves event-free survival in a sub-group analysis of the cardiovascular outcomes in renal atherosclerotic lesions trial. J Vasc Surg 2023;77:1685–92.e2. 10.1016/j.jvs.2022.12.067 [DOI] [PubMed] [Google Scholar]
- 124. Textor SC, Wilcox CS. Renal artery stenosis: a common, treatable cause of renal failure? Annu Rev Med 2001;52:421–42. 10.1146/annurev.med.52.1.421 [DOI] [PubMed] [Google Scholar]
- 125. Rouer M, Godier S, Monnot A et al. Long-term outcomes after transplant renal artery stenosis surgery. Ann Vasc Surg 2019;54:261–8. 10.1016/j.avsg.2018.05.066 [DOI] [PubMed] [Google Scholar]
- 126. Nicholson ML, Yong C, Trotter PB et al. Risk factors for transplant renal artery stenosis after live donor transplantation. Br J Surg 2019;106:199–205. 10.1002/bjs.10997 [DOI] [PubMed] [Google Scholar]
- 127. Patel U, Kumar S, Johnson OW et al. Long-term graft and patient survival after percutaneous angioplasty or arterial stent placement for transplant renal artery stenosis: a 21-year matched cohort study. Radiology 2019;290:555–63. 10.1148/radiol.2018181320 [DOI] [PubMed] [Google Scholar]
- 128. Halimi JM, Al-Najjar A, Buchler M et al. Transplant renal artery stenosis: potential role of ischemia/reperfusion injury and long-term outcome following angioplasty. J Urol 1999;161:28–32. 10.1016/S0022-5347(01)62051-2 [DOI] [PubMed] [Google Scholar]
- 129. Marini M, Fernandez-Rivera C, Cao I et al. Treatment of transplant renal artery stenosis by percutaneous transluminal angioplasty and/or stenting: study in 63 patients in a single institution. Transplant Proc 2011;43:2205–7. 10.1016/j.transproceed.2011.06.049 [DOI] [PubMed] [Google Scholar]
- 130. Wongpraparut N, Chaipruckmalakarn T, Tongdee T et al. Long-term outcome of percutaneous transluminal renal angioplasty (PTRA) versus PTRA with stenting (PTRAS) in transplant renal artery stenosis. BMC Cardiovasc Disord 2021;21:212. 10.1186/s12872-021-02015-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Mousa AY, Broce M, Campbell J et al. Clopidogrel use before renal artery angioplasty with/without stent placement resulted in tertiary procedure risk reduction. J Vasc Surg 2012;56:416–23. 10.1016/j.jvs.2012.01.027 [DOI] [PubMed] [Google Scholar]
- 132. Ivanovic V, McKusick MA, Johnson CM et al. Renal artery stent placement: complications at a single tertiary care center. J Vasc Interv Radiol 2003;14:217–25. 10.1097/01.RVI.0000058324.82956.2a [DOI] [PubMed] [Google Scholar]
- 133. Hiramoto J, Hansen KJ, Pan XM et al. Atheroemboli during renal artery angioplasty: an ex vivo study. J Vasc Surg 2005;41:1026–30. 10.1016/j.jvs.2005.02.042 [DOI] [PubMed] [Google Scholar]
- 134. Misra S, Gomes MT, Mathew V et al. Embolic protection devices in patients with renal artery stenosis with chronic renal insufficiency: a clinical study. J Vasc Interv Radiol 2008;19:1639–45. 10.1016/j.jvir.2008.08.002 [DOI] [PubMed] [Google Scholar]
- 135. Thatipelli MR, Misra S, Sanikommu SR et al. Embolic protection device use in renal artery stent placement. J Vasc Interv Radiol 2009;20:580–6. 10.1016/j.jvir.2009.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Holden A, Hill A. Renal angioplasty and stenting with distal protection of the main renal artery in ischemic nephropathy: early experience. J Vasc Surg 2003;38:962–8. 10.1016/S0741-5214(03)00606-2 [DOI] [PubMed] [Google Scholar]
- 137. Holden A, Hill A, Jaff MR et al. Renal artery stent revascularization with embolic protection in patients with ischemic nephropathy. Kidney Int 2006;70:948–55. 10.1038/sj.ki.5001671 [DOI] [PubMed] [Google Scholar]
- 138. Henry M, Henry I, Klonaris C et al. Renal angioplasty and stenting under protection: the way for the future? Catheter Cardiovasc Interv 2003;60:299–312. 10.1002/ccd.10669 [DOI] [PubMed] [Google Scholar]
- 139. Cooper CJ, Haller ST, Colyer W et al. Embolic protection and platelet inhibition during renal artery stenting. Circulation 2008;117:2752–60. 10.1161/CIRCULATIONAHA.107.730259 [DOI] [PubMed] [Google Scholar]
- 140. Marenzi G, Assanelli E, Marana I et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med 2006;354:2773–82. 10.1056/NEJMoa054209 [DOI] [PubMed] [Google Scholar]
- 141. Solomon R. Hydration: intravenous and oral: approaches, principals, and differing regimens: is it what goes in or what comes out that is important? Interv Cardiol Clin 2020;9:385–93. [DOI] [PubMed] [Google Scholar]
- 142. Soomro QH, Anand ST, Weisbord SD et al. The relationship between rate and volume of intravenous fluid administration and kidney outcomes after angiography. Clin J Amer Soc Nephrol 2022;17:1446–56. 10.2215/CJN.02160222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Abela R, Ivanova S, Lidder S et al. An analysis comparing open surgical and endovascular treatment of atherosclerotic renal artery stenosis. Eur J Vasc Endovasc Surg 2009;38:666–75. 10.1016/j.ejvs.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 144. Kopani K, Liao S, Shaffer K. The coral reef aorta: diagnosis and treatment following CT. Radiol Case Rep 2009;4:209. 10.2484/rcr.v4i1.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Steuer J, Bergqvist D, Björck M. Surgical renovascular reconstruction for renal artery stenosis and aneurysm: long-term durability and survival. Eur J Vasc Endovasc Surg 2019;57:562–8. 10.1016/j.ejvs.2018.09.014 [DOI] [PubMed] [Google Scholar]
- 146. Iwashima Y, Ishimitsu T. How should we define appropriate patients for percutaneous transluminal renal angioplasty treatment? Hypertens Res 2020;43:1015–27. 10.1038/s41440-020-0496-z [DOI] [PubMed] [Google Scholar]
- 147. Boateng FK, Greco BA. Renal artery stenosis: prevalence of, risk factors for, and management of in-stent stenosis. Am J Kidney Dis 2013;61:147–60. 10.1053/j.ajkd.2012.07.025 [DOI] [PubMed] [Google Scholar]
- 148. Lederman RJ, Mendelsohn FO, Santos R et al. Primary renal artery stenting: characteristics and outcomes after 363 procedures. Am Heart J 2001;142:314–23. 10.1067/mhj.2001.116958 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.