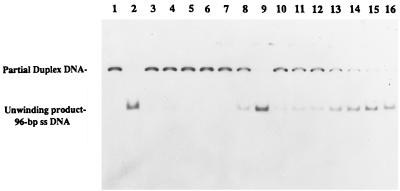
FIG. 8.
UvrD303 protein exhibits a higher helicase activity in unwinding partial duplex DNA substrate. Helicase assays were performed as described in Materials and Methods with the 96-bp partial duplex DNA. Reactions were carried out at 37°C. A no-enzyme control was also incubated at the same temperature for the same period of time as the other samples. The fully denatured control was heated at 100°C for 8 min before loading of the samples. Lanes: 1, no-UvrD control; 2, fully heat-denatured DNA; 3 to 9 (UvrD+), 0.32, 0.8, 1.6, 3.2, 6.4, 8.0, and 32 ng, respectively; 10 to 16 (UvrD303), 0.16, 0.32, 0.8, 1.6, 2.4, 3.2, and 6.4 ng, respectively.