Abstract
Nucleic acid sensing is involved in viral infections, immune response-related diseases, and therapeutics. Based on the composition of nucleic acids, nucleic acid sensors are defined as DNA or RNA sensors. Pathogen-associated nucleic acids are recognized by membrane-bound and intracellular receptors, known as pattern recognition receptors (PRRs), which induce innate immune-mediated antiviral responses. PRR activation is tightly regulated to eliminate infections and prevent abnormal or excessive immune responses. Nucleic acid sensing is an essential mechanism in tumor immunotherapy and gene therapies that target cancer and infectious diseases through genetically engineered immune cells or therapeutic nucleic acids. Nucleic acid sensing supports immune cells in priming desirable immune responses during tumor treatment. Recent studies have shown that nucleic acid sensing affects the efficiency of gene therapy by inhibiting translation. Suppression of innate immunity induced by nucleic acid sensing through small-molecule inhibitors, virus-derived proteins, and chemical modifications offers a potential therapeutic strategy. Herein, we review the mechanisms and regulation of nucleic acid sensing, specifically covering recent advances. Furthermore, we summarize and discuss recent research progress regarding the different effects of nucleic acid sensing on therapeutic efficacy. This study provides insights for the application of nucleic acid sensing in therapy.
Subject terms: Innate immunity, Immunotherapy
Advancements in tumor immunotherapy: the power of nucleic acid sensing
The study highlights the role of nucleic acid sensors in recognizing and destroying microbes, and their potential as targets for cancer therapy and autoimmune disease treatment. Researchers found that modifying these sensors can improve the efficiency of mRNA vaccines and reduce their immunogenicity. Additionally, understanding the crosstalk between different nucleic acid sensing pathways can help develop new optimization strategies for cancer immunotherapy. These findings pave the way for novel therapeutic approaches that harness the power of the immune system to combat various diseases.
This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
Introduction
As a complementary host defense mechanism, the vertebrate immune system consists of both innate and adaptive immune responses1. Although innate immunity cannot confer specificity for host defense or form immune memory, its defense mechanisms can recognize and destroy most microbes within minutes to hours. Cells detect external components of pathogens, or pathogen-associated molecular patterns (PAMPs), through pattern recognition receptors (PRRs), which largely comprise Toll-like receptors (TLRs) and C-type lectin receptors (CLRs). In addition, endosomal TLRs and cytoplasmic nucleic acid receptors, including nucleotide-binding and oligomerization domain NOD-like receptors (NLRs), retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs), absent in melanoma 2 (AIM2)-like receptors (ALRs), and cyclic GMP-AMP synthase (cGAS), recognize cell-invading exogenous nucleic acids; adaptive immune cells are among those that can detect exogenous pathogens via these receptors.
Nucleic acids, which are the genetic building blocks of all organisms, are potent PAMPs released during viral infection and are discerned as exogenous nucleic acids by specialized PRRs. Based on their different forms of nucleic acids, pathogen-derived double-stranded RNA (dsRNA), single-stranded RNA (ssRNA), and DNA are recognized by TLR3, TLR7/8, and TLR9, respectively, in human endosomes. In contrast, nucleic acid (NA)-sensing mechanisms in the cytoplasm contribute to immunity mainly by recognizing invading RNA by RLRs and invading DNA by cGAS and interferon gamma-inducible 16 (IFI16). Activated NA-sensing PRRs transduce signals to aptamer molecules or directly recruit downstream proteins that mediate cytokine and type I and III interferon (IFN) production by activating nuclear factor (NF)-κB and interferon regulatory factor (IRF) proteins, respectively2.
Nucleic acid sensors not only mediate immune defense against pathogens but also detect tumor-derived DNA to trigger antitumor immune responses. Therefore, nucleic acid receptors are potential targets for cancer therapy3,4. Organismal development and aging are accompanied by apoptosis, through which nucleic acids are released from cells5. Many inflammatory and autoimmune diseases are associated with the dysfunctional or abnormal activation of nucleic acid receptors, which are considered attractive targets for the development of therapeutic agonists or antagonists6. To maintain homeostasis and induce optimal immune responses, multiple mechanisms regulate the NA-sensing factors that distinguish between self- and non-self-derived nucleic acids7,8. Nucleic acid sensors have exhibited considerable potential in immunotherapy and the treatment of autoimmune diseases; however, the mechanisms underlying their modulatory roles are unclear. For a long time, innate immune-activating molecules were used as adjuvants in vaccines9; however, the immunogenicity of mRNA has been found to be the main factor diminishing the efficiency of mRNA vaccines10. Therefore, greater attention is being paid to the development of nucleic acid vaccines that do not activate innate immunity and produce more antigenic proteins. In this review, we discuss recent advances in understanding the mechanisms and regulation of NA-sensing and related signaling in different treatments. To highlight the clinical implications of NA-sensing mechanisms, we outline some ways of evading NA-sensing pathways during therapy.
Nucleic acid sensing in endosomes
TLRs constitute a class of transmembrane innate immune receptors that are evolutionarily conserved and induce immune responses by recognizing distinctive PAMPs. TLRs are single-transmembrane proteins composed of an extracellular N-terminal domain, which recognizes ligands; a transmembrane domain; and a cytoplasmic Toll/interleukin 1 receptor (TIR) domain. Human NA-sensing TLRs include TLR3, TLR7, TLR8, and TLR9, which localize to the intracellular compartment membranes and recognize viral and bacterial cytosolic components, such as nonmethylated CpG DNA and single- and double-stranded RNA.
Structure and ligands of nucleic acid-sensing TLRs
TLR3, the first described viral TLR, recognizes dsRNAs larger than 40 bp, which are released during RNA virus replication11. TLR3-induced responses increase in intensity with increasing dsRNA sequence length; however, the underlying molecular mechanism underlying this increase remains unclear12. Two monomeric forms of TLR in solution bind to the dsRNA ligand to form a dimer; the dimerized TLR3 clamps around the dsRNA without any detectable sequence affinity specificity13. In contrast to other NA-sensing TLRs, TLR3 is expressed in immune cells as well as some nonimmune cells, such as neurons and keratinocytes14, and its widespread expression enables it to play a crucial role in RNA virus infection.
TLR7 and TLR8 specifically recognize ssRNA in endosomes. TLR7 and TLR8 preferentially bind guanosine and uridine, respectively, but contain other ssRNA-binding sites15. TLR9 recognizes ssDNA containing unmethylated CpG sequences (commonly found in bacteria and viruses). TLR9 harbors two DNA-binding sites—CpG- and 5′-xCx-binding sites16. RNA:DNA hybrids are also recognized by TLR917.
Trafficking and activation of nucleic acid-sensing TLRs
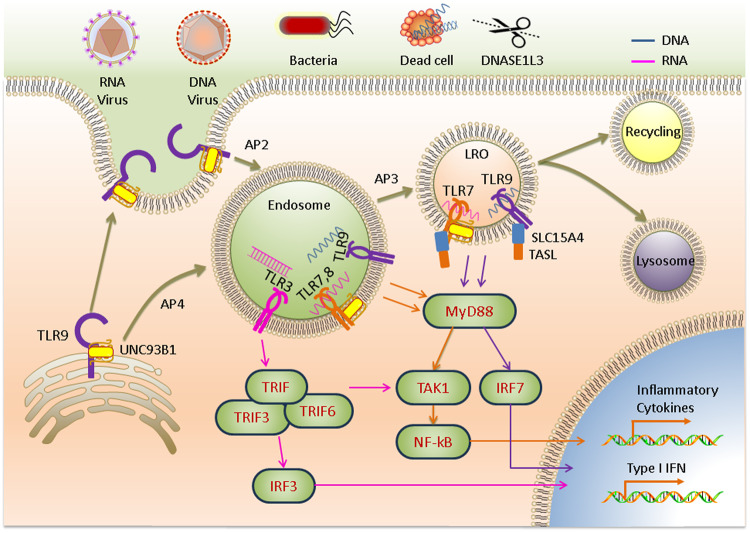
All NA-sensing TLRs are synthesized in the endoplasmic reticulum (ER) and transported to endosomes via the canonical secretory pathway18. However, the characteristics of the transport routes and compartments in which they ultimately reside are surprisingly diverse19. Unc93B1, an ER multiple transmembrane protein, is an essential trafficking molecule for all NA-sensing TLR proteins20 that mediates the differential transport of TLRs21. Inactive TLR9 is native to the ER of dendritic cells (DCs) and B cells, from which it is transported first to the cytoplasmic membrane and then internalized into endosomes via adaptor protein 2 (AP2)-mediated endocytosis22, whereas TLR7 recruits AP4 directly for subsequent translocation to endosomes22 (Fig. 1). TLRs in endosomes undergo proteolytic cleavage, thereby producing functional receptors that interact with nucleic acid ligands23.
Fig. 1. Trafficking of NA-sensing TLRs and downstream signals induced by nucleic acid recognition in endosomes.
TLR9 is first trafficked to the plasma membrane and then internalized into the endosome via AP2, where TRIF is recruited to activate downstream transcription factors. TLR7 and TLR9 depend on AP4 for localization to the endosome to activate the TAK1 signaling pathway via the recruitment of MyD88. AP3 further mediates TLR localization to lysosome-related organelles (LROs), where type I IFN gene activation is mediated.
Activation of all NA-sensing TLRs is restricted to endosomes19. This recognition pattern allows cells to recognize and sequester pathogens in the endosomal compartment without risking infection, and the contents are subsequently sorted for degradation or recycling in a small GTPase-dependent manner. Pathogens enter an endosome via endocytosis. After binding to nucleic acids, a TLR forms a complex, either it’s a hetero or homodimer, and the intracellular TIR domains of the dimerized TLRs come into close contact with each other to activate downstream signal transduction. The signaling cascade depends on the types of ligands, interacting TLRs, and downstream bridging molecules. TLR3 homodimers directly recruit TRIF in response to viral dsRNA binding. Other NA-sensing TLRs trigger the NF-κB and/or IRF signaling pathways via MyD88 to induce cytokine and type I IFN production, promoting inflammatory and antiviral responses, respectively24. TIR domain-containing adaptor-inducing interferon-β (TRIF), TNF-receptor-associated factor 3 (TRAF3), and TRAF6 form a complex that activates IRF3 signaling to produce type I IFN21. However, TLR7 and TLR9 are dependent on AP3-based transport from the endosome to lysosome-related organelles, which is regulated by the peptide transporter protein solute carrier family 15 member 4 (SLC15A4) in the endosomal compartment25. TLR7, TLR8, and TLR9 interact with the TLR adaptor TASL in a lysosomal SLC15A4-dependent manner and activate IRF signaling to produce type I IFN14,26 (Fig. 1).
Because nucleic acids can be derived from various sources, the regulation of TLR ligand availability is essential to balance the pathogen-sensing and self-recognition abilities of TLRs and modulate inflammatory responses, which primarily involve ligand internalization, nucleic acid digestion or processing, and the cytoplasmic transport of ligands.
Regulation of nucleic acid-sensing TLRs
Nucleic acid digestion by nucleases regulates ligand availability. Generally, ligand digestion in the endosomal compartment negatively regulates TLR responses, preventing the generation of autoimmune responses and the excessive activation of antiviral innate immune responses. Nucleases that play a regulatory role in the activation of TLRs include ribonuclease (RNase) T2, deoxyribonuclease (DNase) I-like 3 (DNASE1L3), DNase II, phospholipase D3 (PLD3), and PLD414. RNase T2 is widely expressed in a variety of cell types and negatively regulates TLR3 activation by degrading RNA in endosomal compartments; moreover, it is required for the activation of TLR7 and TLR827,28. RNase T2 deficiency or mutations can cause cystic leukoencephalopathy29. The endonuclease DNase I-like 3 is expressed in innate immune cells and degrades nucleic acids carried by dead cells before it is internalized30 (Fig. 1). DNASE1L3 possesses a unique positively charged and highly hydrophobic C-terminal domain (CTD) that allows it to digest DNA bound to proteins or lipids, which likely contributes to cell transfection difficulties31. Functional mutations in the DNASE1L3 gene cause a rare form of pediatric systemic lupus erythematosus (SLE)32. DNase II degrades DNA in the endosomal compartment, while loss-of-function mutations in DNASE2 cause type I interferonopathies33. PLD3 and PLD4 degrade TLR7 and TLR9 ligands in endolysosomes. Mice deficient in PLD3 and PLD4 suffer from fatal diseases during the early stages of life34. Nuclease deficiency can cause large amounts of nucleic acids to enter the cytoplasm during subsequent endosome rupture, activating the cytosolic NA-sensing pathway and causing type I interferonopathies, which can be alleviated by eliminating type I IFN or blocking TLR trafficking35. The physiological characteristics of some nucleases that play partial roles or are functionally redundant, such as RNase A and DNase I, require further study36.
The amount of ligand internalized by cells is another critical factor affecting ligand availability. It has been shown that the uptake of extracellular immune complexes containing self-nucleic acids is associated with receptor for advanced glycosylation end products (RAGE)37. Self-nucleic acids interact with the antimicrobial peptide LL37 or HMGB1 to promote endosomal uptake of nucleic acids and reduce nuclease degradation, which in turn stimulates the activation of NA-sensing TLRs via self-nucleic acids38,39. The transport of ligands from the nuclear endosome to the cytoplasm can reduce the concentration of ligands in the endosome. SIDT1 and SIDT2 can promote dsRNA escape from the endosome into the cytoplasm and activate antiviral immune signaling40,41.
RNA sensing in the cytosol
Notably, NA-sensing TLRs are mostly expressed in immune cells. However, epithelial cells and fibroblasts on the mucosal surface, which are exposed to the external environment and are susceptible to infection, can still produce an effective innate immune response to prevent pathogen proliferation42. Different cell types employ different nucleic acid recognition mechanisms to combat viral invasion43. Various cytoplasmic RNA-sensing mechanisms have been identified.
Structure and ligands of RLRs
RLRs have been extensively studied as primary cytoplasmic RNA-monitoring mechanisms. RLRs constitute a class of cytoplasmic RNA helicases that detect viral RNA accumulated during infection or replication in a nonsequence-specific manner and elicit antiviral immune responses through the production of type I IFN44,45. In contrast to TLRs, RLRs are expressed by most cell types. The RLR family includes RIG-I, melanoma differentiation-associated gene-5 (MDA-5), and laboratory of genetics and physiology 2 (LGP2). All RLRs have conserved structural domains and contain a central DExD/H-box helicase and CTD. RIG-I and MDA5 also carry two N-terminal caspase recruitment domains (CARDs) that are primarily responsible for signal transduction. In the inactivated state of RIG-I, the CARDs interact with the helicase domain to maintain an autoinhibited conformation. Downstream signaling is initiated by exposure to CARDs when RNA binds to the helicase domain and CTD. This conformational change is thought to be triggered by a V-shaped pincer domain consisting of a unique elbow-shaped helical extension of the CTD with the HEL2 helicase domain46.
RIG-I recognizes the 5′-ppp structure of an RNA and the blunt base-paired 5′ end. DsRNA are characterized by these ligand structures. Some RNA secondary structures consist of the genetic material of many RNA viruses that are generally not found in healthy host cells. In addition, RIG-I can be induced to produce a weaker signal by RNA without the 5′-PPP structure47. Some differences between RLRs have been described48. RIG-I recognizes relatively short dsRNAs, while the ligand preferences of MDA5 have not be fully elucidated; however, it is generally believed that MDA5 preferentially binds to long dsRNAs (>1 kb)49. The open C-shaped structure of MDA5 confers the ability to assemble filamentous oligomers along long dsRNAs50. LGP2 can bind dsRNA; however, it is thought to regulate RLRs because it lacks an NA-sensing signaling function.
Activation of RLRs
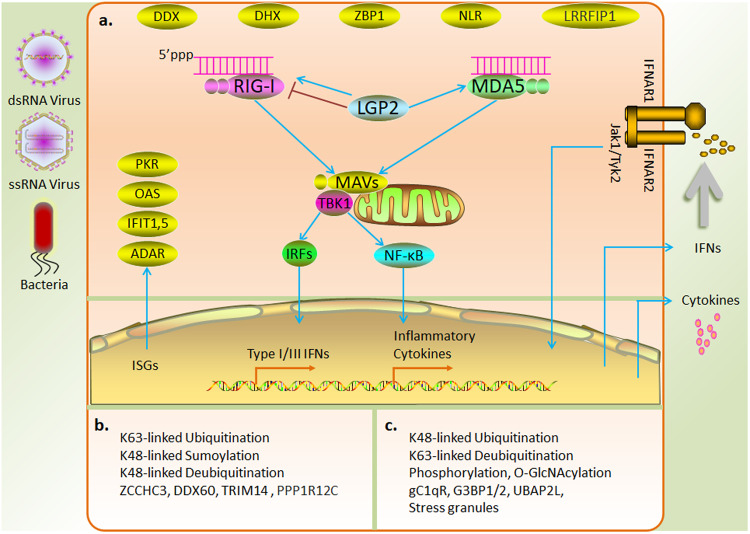
RLRs exposed to CARDs are fully activated by the action of various enzymes and subsequently depend on interactions with 14-3-3ε51 and 14-3-3η52, which are members of the 14-3-3 protein family, to mediate the relocalization of RIG-I and MDA5, respectively, to mitochondria. Mitochondrial antiviral-signaling (MAVS) protein is a common adapter protein associated with RIG-I and MDA5 and is localized to the inner mitochondrial membrane. RLRs interact with the homologous CARD of MAVS and subsequently induce TRAF-binding motifs to recruit TRAF2, TRAF5, TRAF6, and TRADD, which mediate the activation of IRF3 or IRF7 via the action of the cytoplasmic kinase TANK-binding kinase 1 (TBK1) to produce type I and III IFNs15. In addition, MAVS signaling mediates the stimulation of proinflammatory cytokines through the induction of NF-κB activation via the IKK complex53 (Fig. 2a).
Fig. 2. Mechanism and regulation of RNA sensing in the cytosol.
a RLRs are activated by RNA derived from a virus or bacteria and mediate the production of type I and III IFNs and inflammatory cytokines via the MAVS adaptor protein. Interferons released into the extracellular compartment activate interferon-stimulated genes and induce direct antiviral responses. b Positive regulation of RLRs by posttranslational modifications and interacting proteins. c Negative regulation of RLRs by posttranslational modifications and interacting proteins.
LGP2, which lacks a signaling structural domain, has been shown to regulate RIG-I and MDA5 in several studies. LGP2 inhibits RIG-I activation through ligand competition54 or by directly impeding the oligomerization and signal activation of RIG-I, which is mediated through the RIG-I CTD domain55. In addition, LGP2 interacts with tripartite motif-containing 25 (TRIM25) to inhibit RIG-I ubiquitination56. In contrast, LGP2 facilitates MDA5 signaling57,58. During viral infection, LGP2 has also shown to promote both RIG1 and MDA5 signaling59 (Fig. 2a). In conclusion, the characterization of the regulatory role of LGP2 under specific physiological conditions requires further study.
Regulation of RLRs
In addition to LGP2, multiple intracellular mechanisms participate in the regulation of RLR activity, including multiple posttranslational modifications (PTMs) and protein interactions. Ubiquitination of RIG-I CARD via K63 linkages, mediated by the ubiquitinated proteins TRIM25, Riplet, TRIM4, and Mex-3 RNA-binding family member C (Mex3c), promotes RIG-I oligomerization and signal transduction60–63; in contrast, polyubiquitination via K48 linkages, mediated by ring finger protein 122 (RNF122), RNF125, Casitas B-lineage lymphoma (c-Cbl), and TRIM4064, induces RIG-I degradation. Deubiquitinases, including ubiquitin-specific peptidase 3 (USP3), USP21, and CYLD lysine 63 deubiquitinase (CYLD), attenuate the antiviral response by removing the K63-linked polyubiquitin chain. In contrast, USP4 and USP15 enhance the stability of RIG-I by hydrolyzing the K48-linked ubiquitin chain and exerting a positive regulatory effect65. SUMOylation prevents RLR degradation via K48-polyubiquitin-dependent degradation, thereby stabilizing RLR in the early stages of viral infection66. Additionally, phosphorylation causes RIG-I to be autoinhibited67. A recent study showed that O-GlcNAcylation inhibited RIG-1 signaling by modifying MAVS68 (Fig. 2b, c). Although the PTMs related to MDA5 signaling have been studied relatively rarely, it is likely that PTMs regulate MDA5 in a manner similar to their regulation of RIG-I.
Many dsRNA-binding proteins participate in the regulation of RLRs. PACT positively regulates RLRs by interacting with the CTD of RIG-I or promoting MDA5 oligomerization69,70. The zinc finger protein ZCCHC3 has recently been shown to function as a coreceptor for RIG-I and MDA571. DExD/H-box helicase 60 (DDX60) promotes RIG-I-dependent innate immune responses72. In addition to covalent modifications, TRIM14 enhanced RIG-I signaling by recruiting NF-κB essential regulator (NEMO) to the MAVS complex via the ubiquitin chain73. A recent study demonstrated that PPP1R12C relocalization triggered by viral infection or RNA delivery reagents promoted downstream signaling by mediating the dephosphorylation of RLRs74. In contrast, the complement component C1q (gC1qR) on mitochondria inhibited RIG-I- and MDA5-dependent antiviral responses75. Stress granules formed by the aggregation of the key nucleating factors G3BP1/2 and UBAP2L with stalled ribosome–mRNA complexes inhibited excessive activation of RLR signaling and prevented viral replication through unknown physiological functions76 (Fig. 2b, c).
Other RNA sensors
Several other cytoplasmic RNA sensors trigger antiviral responses via transcription factors, including certain DExD/H-box RNA helicases (which recognize RNA through their conserved motifs and are involved in the activation of TLR and RLR downstream signaling pathways), NLRs (which induce inflammasome activation by binding RNA), the LRR domain of flightless-1-interacting protein 1 (LRRFIP1, which binds dsRNA and dsDNA to induce type I IFN production through β-catenin phosphorylation), Z-DNA binding protein (ZBP177, which induces activation of innate immunity and PANoptosis through recognition of Z-DNA and Z-RNA) (Fig. 2a), and HMGB (which may act as a cosensor for various PRRs) (see reviews47,78).
Various RNA sensors with direct antiviral activity are expressed in cells; these sensors include 2′,5′-oligoadenylate synthetase (OAS), RNA-regulated protein kinase (PKR), IFN-induced protein with tetratricopeptide repeats 1 (IFIT1), and adenosine deaminase acting on RNA (ADAR), the expression of which depend on type I IFN or PRR signaling7,79 (Fig. 2a). OAS binds dsRNA and catalyzes the generation of 2′-5′-linked oligoadenylates (2–5A) from substrate ATP to degrade virus-derived dsRNA by mediating the activation of RNase L. PKR can be activated by viral-derived dsRNA or short 5′-ppp RNA-containing secondary structures. Activated PKR mediates the phosphorylation of the α-subunit of eukaryotic initiation factor 2 (eIF2) to inhibit translation initiation. IFIT1 binds to ssRNAs containing the 5′-ppp terminus to repress cap-dependent RNA translation. ADAR-edited cell-derived self-RNAs can evade NA-sensors; however, A-I editing may lead to amino acid substitutions and loss of function of viral proteins80.
DNA sensing in the cytosol
Cells infected with a DNA virus but that do not express TLR9 produce high levels of type I IFN81. Therefore, ZBP182 and RNA Pol III83 were initially identified as cytoplasmic DNA sensors. However, subsequent studies revealed that RNA Pol III-mediated innate immune responses were dependent on poly (dA:dT)-converted RNA ligands with 5′-triphosphate and double-stranded secondary structures to activate the RIG-I/MAVS pathway (Fig. 3a), and interferon production was induced in mouse cells lacking MAVS. Similarly, ZBP1 plays a role only in specific cell types, suggesting that DNA activates unknown DNA-sensing pathways in the cytoplasm in a nonsequence-specific manner.
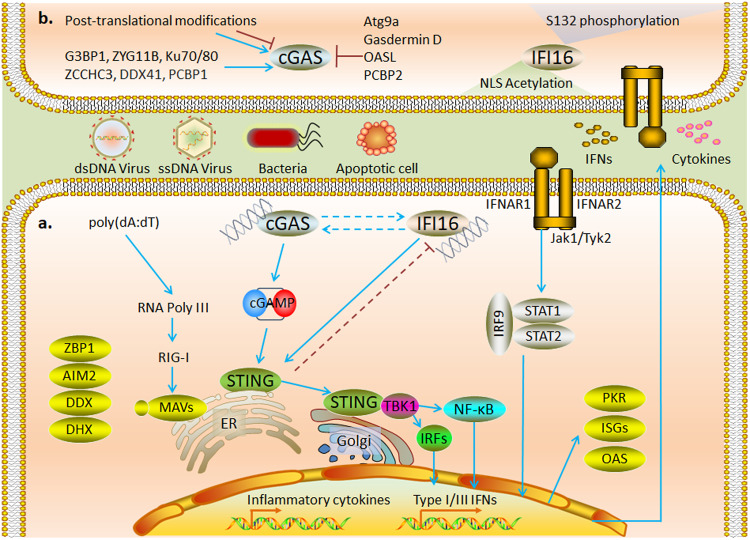
Fig. 3. Mechanism and regulation of DNA sensing in the cytosol.
a cGAS and IFI16, as major DNA receptors in the cytoplasm, induce STING-dependent inflammatory cytokines and IFN production and inhibit viral replication by activating interferon-stimulated genes. b Posttranslational modifications of amino acid residues at different sites regulate the activity of cGAS and nuclear localization of IFI16; a variety of proteins have been shown to participate in regulating the activity of cGAS.
Structure and ligands of cytosolic DNA sensors
Interferon-gamma inducible protein 16 (IFI16) and cGAS have been identified as cytoplasmic DNA receptors. Mammalian cGAS belongs to the cGAS/DncV-like nucleotidyltransferase (CD-NTase) family, the members of which are structurally similar to OAS84. cGAS contains a disordered N-terminus that anchors its inactivated form to the inner cell membrane85, a central NTase domain, and a C-terminal Mab-21 homology domain containing the zinc-ribbon/thumb motif. cGAS binds to dsDNA to form a dimer, followed by DNA sequestration via liquid-phase condensation86. cGAS–DNA condensation protects the DNA against Trex1 nuclease-mediated DNA degradation87. cGAS activation by dsDNA is DNA length dependent88, as more than 45 bp of a dsDNA molecule binding to the A and B sites of each hcGAS molecule and with a third binding site that promotes the stability of the complex.89. In addition, cGAS generates innate immunity by recognizing RNA:DNA hybrid molecules generated by intracellular reverse transcription of the HIV-1 virus90. PQBP1 acts as an intracellular receptor by which HIV cDNA is recognized by cGAS91.
IFI16 (p204 in mice), a member of the ALR family, contains a pyrin structural domain (PYD) and two DNA-binding hematopoietic interferon-inducible nuclear antigens with 200-amino-acid repeat (HIN) structural domains. IFI16 also binds to dsDNA in a length-dependent manner92. When binding dsDNA molecules, the PYD structural domain of IFI16 assembles into filamentous oligomers in synergistic association with neighboring PYDs and induces STING-dependent type I IFN production93. In addition, IFI16 recognizes viral RNA, promotes RIG-I activation through direct interaction, and upregulates RIG-I transcription by recruiting RNA polymerase II, which provides evidence of crosstalk between RNA- and DNA-sensing mechanisms94.
Activation of cytosolic DNA sensors
Activation of cGAS requires nuclear export signals to mediate its cytoplasmic localization95. cGAS catalyzes the generation of the second messenger cGAMP from ATP and GTP, induces IFN production through activation of the STING-TBK1-IRF3 axis, and mediates cytokine production through activation of NF-κB. IFI16 shuttles between the nucleus and cytoplasm and mediates interferon production via a STING-dependent cytosolic signaling pathway92,96 (Fig. 3a). In a sequencing analysis of four cell types, IFI16 was found to exert a crucial effect on the transfection efficiency of plasmid DNA (pDNA)97.
STING is predominantly located on the ER outer membrane and is expressed in most cells. STING mediates the cytoplasmic dsDNA-induced antiviral innate immune response as an adaptor molecule in response to cGAS and IFI16. cGAMP directly binds to STING, induces STING movement from the ER to the Golgi apparatus, and ultimately recruits TBK1 to colocalize with STING puncta in the perinuclear region. TBK1 recruitment is critical for STING-mediated IRF3 and NF-κB activation98. DNA-bound IFI16 interacts with STING in the cytoplasm to recruit and activate TBK1-IRF3 signaling and mediate IFN production99 (Fig. 3a).
Regulation of cytosolic DNA sensors
cGAS is strictly regulated to produce a balanced immune response100. Intracellular nucleases are essential for ligand availability in cytoplasmic DNA sensors. Deficiency or mutation in TREX1, RNASEH2, or SAMHD1 leads to cGAS-dependent type I IFN production101–103. In addition, multiple mechanisms participate in the regulation of the posttranslational modifications of cGAS104. Elimination of the K48-linked ubiquitinated chain suppresses P62-mediated autophagic degradation of cGAS105. However, the abrogation of K63-linked polyubiquitination promotes the DNA-binding ability of cGAS106. Interestingly, the deubiquitinating enzyme OTUD3 promotes cGAS-mediated DNA sensing but inhibits RLR-mediated RNA sensing107. The acetylation of lysine residues in the unstructured N-terminal region of hcGAS promotes its activation108. In contrast, acetylation of Lys384/Lys394/Lys414 inhibited cGAS activation109. SUMOylation at different sites exerts different regulatory effects on cGAS. SENP2-mediated deSUMOylation induces cGAS degradation during late viral infection110. However, SENP7-mediated deSUMOylation enhances cGAS activation111. AKT, CDK1, DNA-PK, and Aurora A-mediated phosphorylation of hcGAS can inhibit its enzymatic activity112–114. O-GlcNAcylation has been reported to regulate NA-sensing in various cells, although the mechanism remains unclear115. OGT has recently been found to activate cGAS-mediated innate immune responses by enhancing the stability of SAMHD1, thereby promoting intracellular dNTP depletion and generating DNA replication intermediates116 (Fig. 3b).
High acetylation and phosphorylation rates of endogenous IFI16 have been found in lymphocytes and are mainly associated with nuclear localization. IFI16 carries a nuclear localization signal (NLS) at the N-terminus, and the NLS motif is modified by acetyltransferase p300 to promote accumulation in the cytoplasm117. In contrast, phosphorylation by CD2 on S132 promotes the nuclear localization of IFI16118. Recent studies revealed that clearly localized IFI16 prevented DNA viral invasion via its effect on different pathways119 (Fig. 3b).
Several proteins have been found to mediate cGAS signaling by interacting with ligands or regulating ligand action. Among these proteins, G3BP1, ZYG11B, Ku, and ZCCHC3 promote cGAS-mediated innate immune responses by facilitating DNA binding and condensation120–123. DEAD-box helicase 41 (DDX41) promotes cGAS activation by regulating DNA stabilization via its helicase activity124. Others, such as Atg9a and Gasdermin D, inhibit STING-dependent innate immune responses by mediating autophagy125,126. OASL suppresses IFN production by specifically binding to cGAS during DNA virus infection127. Notably, poly(rC)-binding protein 1 (PCBP1) facilitates the binding of cGAS to DNA, whereas PCBP2 interacts with cGAS and prevents its excessive activation128,129 (Fig. 3b). Additionally, cGAS, IFI16, and STING regulate each other. cGAS may contribute to the innate immune response by increasing the stability of IFI16130, and IFI16-mediated TBK1 recruitment is essential for cGAMP-mediated STING activation96,131. STING negatively regulates antiviral immune responses through TRIM21-mediated ubiquitinated degradation of IFI16132. Moreover, the transport of extracellular second messenger cyclic dinucleotides (CDNs) by SLC19A1133, SLC19A2134, LRRC8135, LL37136, P2X7R137, and Connexin138 is essential for the activation of intracellular STING. ABCC1 has recently been identified as a cGAMP export protein139. ENPP1 attenuates STING activation in bystander cells by degrading extracellular cGAMP140.
Other cytosolic DNA sensors
Other DNA sensors can recognize DNA in specific cell types or may recognize only specific sequences, mainly, the DExD/H-box helicases DHX9 and DHX36 (which recognize CpG DNA to activate the TLR downstream signaling pathway), DDX41 and DDX60 (which enhance the type I IFN response by binding dsDNA), and AIM2 (which triggers the inflammasome pathway by binding dsDNA to produce IL-1β and IL-18) (see reviews42,141) (Fig. 3a).
Nucleic acid sensing as a promising therapeutic target
NA-sensing exerts both pro- and antitumor effects at different stages of tumorigenesis. Genomic instability typically produces autoimmunogenic DNA in cancer cells. Therefore, NA-sensing-mediated IFN production contributes to DC maturation and tumor-specific T-cell responses142. However, in vitro studies have revealed that NA-sensing pathways in several cancer cells are inhibited by JAK2-STAT3-mediated signaling143. External activation of NA-sensing has shown enhanced antitumor effects in a variety of cancers. However, in metastatic cancer, the cGAS-STING-TBK1 axis-mediated inflammatory response is positively associated with tumor metastasis. These opposing effects may be associated with the type and stage of the tumor8.
NA-sensing-associated mechanisms also play different regulatory roles in gene therapy. Genetic vaccines, including DNA and RNA vaccines consisting the nucleic acids of target genes, are injected directly into the body to induce innate and adaptive immune responses144,145. pDNA is an intrinsic adjuvant for DNA vaccines and is essential for the activation of resident antigen-presenting cells through activation of the innate immune response via the action of STING-TBK1146,147. However, type I IFN produced by activated nucleic acid induction inhibits the translation of mRNA vaccine-encoded antigenic proteins, thereby reducing antigen-specific immunity148,149. Type I IFN is probably critical for enhancing the early immune response but is also the main cause of side effects150. For optimal treatment outcomes, it is essential that the immunostimulation and transfection efficiency of nucleic acids be balanced when designing therapeutic strategies151.
Positive regulation of nucleic acid sensing in therapy
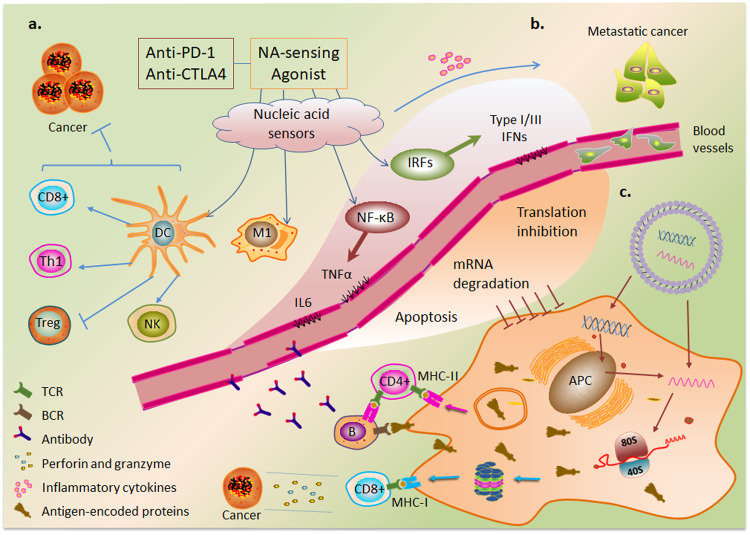
The activation of NA-sensing in cancer cells promotes hot tumor transformation through the production of type I IFN and cytokines152. Type I interferons also upregulate the expression of major histocompatibility complex (MHC) class I molecules in antigen-presenting cells, which present processed cancer cell-derived antigen molecules to CD8+ T cells152. Stimulating the production of type I and III IFNs in CD4+ T cells confers self-protection against HIV infection and enhances the ability of CAR-T cells to clear tumor cells153. In addition, cGAS-mediated cGAMP release from cancer cells activates adjacent immune cells154,155. Studies have shown that the DNA released from tumor cells after chemotherapy and radiotherapy activates NA-sensing signals that synergistically enhance antitumor effects156–158. Agonists of cGAS, STING, and RIG-I potentiate the antitumor activity of immune cells159–161; for example, the combination of a STING agonist and a PD1 blocker showed therapeutic effects in tumors with low immunogenicity162. Similarly, the innate immune response mediated by RIG-I ligands in combination with CTLA-4 blockade enhanced adaptive immune response-mediated antitumor effects163. Furthermore, this combination therapy can enhance the antitumor effect of the anti-PD1 antibody in a cGAS-dependent manner by inhibiting the protein arginine methyltransferase PRMT1- and PRMT5-mediated methylation of the cGAS residues Arg133 and Arg124, respectively164,165 (Fig. 4a). A recent study suggested that inducing RIG-I-dependent OAS/RNase L-mediated apoptosis is a potential strategy for cancer immunotherapy166.
Fig. 4. Differential regulation of NA-sensing signaling pathways in therapy.
a NA-sensing promotes the antitumor therapeutic efficacy of immune checkpoint inhibitors by inducing dendritic cell (DC) maturation and tumor-specific T-cell responses and promotes the differentiation of macrophages into M1 proinflammatory macrophages. b In the metastatic stage of cancer, NA-sensing-induced inflammatory cytokines exhibit cancer-promoting effects. c Model of antigen-specific immunity mediated by nonviral gene therapies and the negative regulatory effects of NA-sensing on therapeutic transgenes.
Because the basic components of pDNA, such as the TLR9 agonist, are immunogenic, the unmethylated CpG sequence is commonly used as a vaccine adjuvant167. CpG oligodeoxynucleotides (ODNs) stimulate the maturation and survival of plasmacytoid DCs and accelerate regulatory T (Treg) cell differentiation and depletion through the activation of TLR9168,169. Recent studies revealed that SARS CoV-2 mRNA vaccination exposes HIV to CD8+ T cells170. A small-molecule agonist of RIG-I, KIN1148, exhibits an adjuvant effect on influenza virus vaccine immunity171. The dsRNA analog poly(I:C) activates TLR3 and MDA5 to induce Th1 cell and CD8+ T-cell immune responses through the production of IFN and cytokines172. Activation of TLR7/8 and the RIG-I pathway promotes macrophage differentiation toward the M1 proinflammatory phenotype and exhibits antitumor activity173,174 (Fig. 4a). In summary, NA-sensing has emerged as a promising target for cancer immunotherapy175.
Negative regulation of nucleic acid sensing in therapy
The disadvantages of intrinsic NA-sensing activation are mainly observed in autoimmune and inflammatory diseases176. NA-sensing is especially important during the metastatic stage of cancer and is activated under specific conditions. Increased levels of inflammatory factors caused by NA-sensing have been associated with poor prognosis8 (Fig. 4b). Recent studies have shown that RIG-I attenuates the tumor-killing effect of CD8+ T cells by inhibiting STAT5 action177.
Although the innate immune response induced via nucleic acid immunity can contribute to disease attenuation, it also plays a negative regulatory role. NA-sensing induces apoptosis in host cells via multiple pathways178. IRF3-mediated apoptosis impairs T-cell proliferation and metabolism179. Mechanistically, activated IRF3 binds to the proapoptotic protein Bax, and the subsequent translocation of the IRF3-Bax complex to mitochondria promotes the release of cytochrome c into the cytoplasm, thereby inducing apoptosis8,180. In contrast, NA-sensing mediates the degradation of transfected RNA and inhibits translation initiation through the actions of interferons181. OAS recognizes dsRNA and activates RNase L to mediate RNA degradation. The degraded RNA can also activate other NA-sensing PRRs182. dsRNA-dependent activation of PKR subsequently phosphorylates translation initiation factor eIF2α, resulting in translation repression183. IFIT1 can also suppress translation by sequestering eukaryotic initiation factors or directly binding to the 5′ end of foreign RNA184 (Fig. 4c).
mRNA vaccines elicit different immune responses by encoding antigenic proteins. On the one hand, mRNA-encoded proteins acting as endogenous antigens are degraded by proteasomes into antigenic peptides and activate CD8+ T cells via MHC class I molecules. On the other hand, mRNA vaccine-encoded proteins secreted into extracellular compartments are internalized by antigen-presenting cells, which generate antigenic peptides by proteolysis in endosomes and are presented to CD4+ T cells via MHC class II molecules, which can induce cytokine secretion and stimulate B cells to activate humoral immune responses185 (Fig. 4c). The induction of these immune responses depends on the transfection efficiency of the mRNA vaccine and is inhibited mainly by negative regulatory effects mediated by NA-sensing149. NA-sensing also causes gene editing difficulty in some cells. Inhibition or evasion of NA-sensing can save nucleic acids from translational repression, thereby improving gene transfection efficiency and increasing the expression of functional protein products186,187.
Strategies to evade nucleic acid sensing
Small-molecule inhibitors and viral proteins
Small-molecule inhibitors (see review188) of DNA sensing pathways have potential therapeutic value in diseases with long-term activation of proinflammatory pathways, such as autoimmune and inflammatory diseases161,189,190. In addition, A151 ODN inhibits the activity of multiple DNA receptors191, and 2′-O-methyl (2′OMe) gapmer-modified antisense oligonucleotides show sequence-dependent inhibition of NA-sensing mediated via RNase-H1 recruitment192.
Understanding how viruses evade immune recognition is important for antiviral research and immunotherapy193. Multiple virus-encoded proteins inhibit NA-sensing-associated pathways (see review194). Vaccinia virus (VACV), the most studied Poxviridae195, degrades cGAMP via B2R gene-encoded POXIN196. Some viruses are thought to improve the efficiency of nucleic acid vaccines by blocking the RNA-sensing pathway and enhancing gene expression197. Among these proteins, influenza A virus nonstructural protein 1 (NS1) stimulates mRNA translation by inhibiting interferon production198. Vaccinia protein B18R inhibits type I IFN to enhance mRNA stability and translation efficiency199.
Sequence optimization and chemical modifications
Nucleic acid modification can prevent the innate immune response-mediated translational repression of exogenous genes by reducing immunogenicity186. The 5′-cap1 structure (a 2′-O-methyl group linked to the first nucleotide: m7GpppNmpN) can escape RIG-I recognition, thereby increasing translation efficiency200,201. The addition of poly(A) tails minimizes mRNA immunogenicity by reducing the U content of the sequence186. Circular RNAs (circRNAs) reportedly exhibit low immunogenicity and high stability and can initiate stable translation via internal ribosome entry site elements202,203. The incorporation of N6-methyladenosine (m6A)-modified circRNAs completely abrogated RIG-I-mediated activation of the immune response204. In addition, many chemical modifications of RNA bases have been leveraged to reduce the immunogenicity of mRNA; these modifications include pseudouridine (Ψ), N1-methyl-pseudouridine (m1Ψ), 2-thiouridine (s2U), 5-methoxyuridine (m5U), and 5-methylcytidine (m5C)205–207. DNA transfection was performed to construct CAR-modified immune cells, and the low efficiency of pDNA transfection in immune cells was appropriately resolved by removing CpG sequences and reducing plasmid size208.
Limitations and prospects of nucleic acid sensing in therapy
Despite multiple modifications aimed at limiting undesired immune stimulation caused by nucleic acid vaccines, further optimization is necessary to achieve the desired transfection efficiency and economic viability. For instance, although DNA vaccines can trigger an immune response in animal experiments, they exhibit low immunogenicity in human clinical trials147, thereby slowing the development of DNA vaccines. DNA vaccines have also been used in the fight against COVID-19; for example, the COVIDITY DNA vaccine was developed with two plasmids encoding the S protein receptor-binding domain and the nucleocapsid (N) protein, thus providing a mechanism to enhance extracellular antigen cross-presentation. Nevertheless, although physical delivery methods such as electroporation or needle-free injection systems may address delivery efficiency issues, the risk of DNA insertion remains a concern209. In contrast, agonists and antagonists of DNA sensors show more promise for clinical applications6. In contrast to DNA vaccines, RNA vaccines carry no risk of genomic insertion and are easy to deliver. Although balancing antigen expression and immunogenicity of RNA can increase the antigen availability, the thermal instability of these vaccines remains a challenge that has not been adequately addressed.
As mentioned earlier, NA-sensing activation exhibits both benefits and drawbacks in disease treatment, and its necessity must be carefully evaluated in the context of different diseases and stages of pathogenesis. The study and comparison of DNA-sensing and RNA-sensing interactions can help in identifying new optimization strategies210,211. The low immunogenicity of DNA vaccines may be due to some degree of cell-type specificity of DNA sensors, but it is unclear where nucleic acid vaccines that are injected into the skin accumulate. Targeted delivery of nucleic acid vaccines to lymph nodes or tumors may reduce NA-sensing while enhancing antigen-specific immune responses212. Furthermore, the effect of STING on tumor-associated macrophage differentiation helps alleviate tumor cell-mediated immunosuppression in the tumor microenvironment213. An alternative method for engineering T cells is in vivo RNA transfection214, although the role of NA-sensing of in vitro transcribed mRNA after CAR transfection remains unclear. A vast body of research links NA-sensing modulation to other therapeutic approaches215.
Conclusions
NA-sensing plays an important role in immunotherapy owing to its ability to elicit innate immunity. Therefore, a comprehensive understanding of the regulation and mechanisms underlying NA-sensing may contribute to the development of antitumor therapies. Several emerging regulatory mechanisms complement the profiling of NA-sensing systems. Although human nucleic acid receptors are diverse, their recognition ligands overlap, and there are similarities in their regulatory mechanisms and downstream signals, such as common adaptor proteins and cofactors. To effectively prevent pathogenic infections, humans have evolved redundant NA-sensing systems to complement the cellular recognition of immunogenic nucleic acids. Therefore, crosstalk among nucleic acid receptors is essential216. In this review, we describe the regulatory mechanisms of nucleic acid receptors.
NA-sensing is a double-edged sword in the field of therapeutics. In cancer therapy, NA-sensing tends to have a facilitative effect on antitumor immunity and is thus considered a potential treatment target. However, in the field of gene therapy, it is important to prevent the excessive activation of NA-sensing pathways to maintain proper immunogenicity and efficient gene transfection. The application of in vitro-transcribed mRNA has emerged as a promising therapeutic strategy. Multiple modification approaches have been proposed for increasing therapeutic efficiency by increasing transfection efficiency. In conclusion, nucleic acid sensors are potential targets for gene and cell therapies, which must be generated to balance therapeutic transgene-mediated innate and adaptive immune responses145,217,218.
Acknowledgements
This work was supported by the National Research Foundation grant (2022M3E5F1016693) and the National Research Council of Science and Technology (NST) grant (CAP-18-02-KRIBB) by the Korean government. We sincerely appreciate laboratory members for helpful discussions in preparing this manuscript.
Author contributions
T.D.K. and L.Z.K. conceived the manuscript. L.Z.K. drafted the manuscript. All authors reviewed the manuscript. S.M.K, C.L.W. modified the manuscript. T.D.K. supervised manuscript preparation. All authors have read and agreed to the published version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marshall JS, Warrington R, Watson W, Kim HL. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018;14:49. doi: 10.1186/s13223-018-0278-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, et al. cGAS-STING pathway in cancer biotherapy. Mol. Cancer. 2020;19:136. doi: 10.1186/s12943-020-01247-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amouzegar, A., Chelvanambi, M., Filderman, J. N., Storkus, W. J. & Luke, J. J. STING agonists as cancer therapeutics. Cancers (Basel)13, 10.3390/cancers13112695 (2021). [DOI] [PMC free article] [PubMed]
- 5.Yang H, Wang H, Ren J, Chen Q, Chen ZJ. cGAS is essential for cellular senescence. Proc. Natl Acad. Sci. USA. 2017;114:E4612–E4620. doi: 10.1073/pnas.1705499114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McWhirter SM, Jefferies CA. Nucleic acid sensors as therapeutic targets for human disease. Immunity. 2020;53:78–97. doi: 10.1016/j.immuni.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Schlee M, Hartmann G. Discriminating self from non-self in nucleic acid sensing. Nat. Rev. Immunol. 2016;16:566–580. doi: 10.1038/nri.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okude H, Ori D, Kawai T. Signaling through nucleic acid sensors and their roles in inflammatory diseases. Front. Immunol. 2020;11:625833. doi: 10.3389/fimmu.2020.625833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kayraklioglu N, Horuluoglu B, Klinman DM. CpG oligonucleotides as vaccine adjuvants. Methods Mol. Biol. 2021;2197:51–85. doi: 10.1007/978-1-0716-0872-2_4. [DOI] [PubMed] [Google Scholar]
- 10.Zhang C, Maruggi G, Shan H, Li J. Advances in mRNA vaccines for infectious diseases. Front. Immunol. 2019;10:594. doi: 10.3389/fimmu.2019.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berke IC, Li Y, Modis Y. Structural basis of innate immune recognition of viral RNA. Cell Microbiol. 2013;15:386–394. doi: 10.1111/cmi.12061. [DOI] [PubMed] [Google Scholar]
- 12.Jelinek I, et al. TLR3-specific double-stranded RNA oligonucleotide adjuvants induce dendritic cell cross-presentation, CTL responses, and antiviral protection. J. Immunol. 2011;186:2422–2429. doi: 10.4049/jimmunol.1002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakaniwa K, et al. TLR3 forms a laterally aligned multimeric complex along double-stranded RNA for efficient signal transduction. Nat. Commun. 2023;14:164. doi: 10.1038/s41467-023-35844-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyake K, et al. Nucleic acid sensing by toll-like receptors in the endosomal compartment. Front. Immunol. 2022;13:941931. doi: 10.3389/fimmu.2022.941931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu G, Gack MU. Distinct and orchestrated functions of RNA sensors in innate immunity. Immunity. 2020;53:26–42. doi: 10.1016/j.immuni.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawasaki T, Kawai T. Discrimination between self and non-self-nucleic acids by the innate immune system. Int Rev. Cell Mol. Biol. 2019;344:1–30. doi: 10.1016/bs.ircmb.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rigby RE, et al. RNA:DNA hybrids are a novel molecular pattern sensed by TLR9. EMBO J. 2014;33:542–558. doi: 10.1002/embj.201386117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Majer O, Liu B, Barton GM. Nucleic acid-sensing TLRs: trafficking and regulation. Curr. Opin. Immunol. 2017;44:26–33. doi: 10.1016/j.coi.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lind NA, Rael VE, Pestal K, Liu B, Barton GM. Regulation of the nucleic acid-sensing Toll-like receptors. Nat. Rev. Immunol. 2022;22:224–235. doi: 10.1038/s41577-021-00577-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452:234–238. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- 21.Lee BL, Barton GM. Trafficking of endosomal Toll-like receptors. Trends Cell Biol. 2014;24:360–369. doi: 10.1016/j.tcb.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee BL, et al. UNC93B1 mediates differential trafficking of endosomal TLRs. Elife. 2013;2:e00291. doi: 10.7554/eLife.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park B, et al. Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat. Immunol. 2008;9:1407–1414. doi: 10.1038/ni.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32:305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Rimann I, et al. The solute carrier SLC15A4 is required for optimal trafficking of nucleic acid-sensing TLRs and ligands to endolysosomes. Proc. Natl Acad. Sci. USA. 2022;119:e2200544119. doi: 10.1073/pnas.2200544119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinz LX, et al. TASL is the SLC15A4-associated adaptor for IRF5 activation by TLR7-9. Nature. 2020;581:316–322. doi: 10.1038/s41586-020-2282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greulich W, et al. TLR8 is a sensor of RNase T2 degradation products. Cell. 2019;179:1264–1275.e1213. doi: 10.1016/j.cell.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu K, et al. Skewed endosomal RNA responses from TLR7 to TLR3 in RNase T2-deficient macrophages. Int. Immunol. 2021;33:479–490. doi: 10.1093/intimm/dxab033. [DOI] [PubMed] [Google Scholar]
- 29.Henneke M, et al. RNASET2-deficient cystic leukoencephalopathy resembles congenital cytomegalovirus brain infection. Nat. Genet. 2009;41:773–775. doi: 10.1038/ng.398. [DOI] [PubMed] [Google Scholar]
- 30.Sisirak V, et al. Digestion of chromatin in apoptotic cell microparticles prevents autoimmunity. Cell. 2016;166:88–101. doi: 10.1016/j.cell.2016.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilber A, Lu M, Schneider MC. Deoxyribonuclease I-like III is an inducible macrophage barrier to liposomal transfection. Mol. Ther. 2002;6:35–42. doi: 10.1006/mthe.2002.0625. [DOI] [PubMed] [Google Scholar]
- 32.Al-Mayouf SM, et al. Loss-of-function variant in DNASE1L3 causes a familial form of systemic lupus erythematosus. Nat. Genet. 2011;43:1186–1188. doi: 10.1038/ng.975. [DOI] [PubMed] [Google Scholar]
- 33.Rodero MP, et al. Type I interferon-mediated autoinflammation due to DNase II deficiency. Nat. Commun. 2017;8:2176. doi: 10.1038/s41467-017-01932-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gavin AL, et al. PLD3 and PLD4 are single-stranded acid exonucleases that regulate endosomal nucleic-acid sensing. Nat. Immunol. 2018;19:942–953. doi: 10.1038/s41590-018-0179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshida H, Okabe Y, Kawane K, Fukuyama H, Nagata S. Lethal anemia caused by interferon-beta produced in mouse embryos carrying undigested DNA. Nat. Immunol. 2005;6:49–56. doi: 10.1038/ni1146. [DOI] [PubMed] [Google Scholar]
- 36.Santa P, et al. The role of nucleases and nucleic acid editing enzymes in the regulation of self-nucleic acid sensing. Front. Immunol. 2021;12:629922. doi: 10.3389/fimmu.2021.629922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertheloot D, et al. RAGE enhances TLR responses through binding and internalization of RNA. J. Immunol. 2016;197:4118–4126. doi: 10.4049/jimmunol.1502169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ganguly D, et al. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J. Exp. Med. 2009;206:1983–1994. doi: 10.1084/jem.20090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ivanov S, et al. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood. 2007;110:1970–1981. doi: 10.1182/blood-2006-09-044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen TA, et al. SIDT1 localizes to endolysosomes and mediates double-stranded RNA transport into the cytoplasm. J. Immunol. 2019;202:3483–3492. doi: 10.4049/jimmunol.1801369. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen TA, et al. SIDT2 transports extracellular dsRNA into the cytoplasm for innate immune recognition. Immunity. 2017;47:498–509.e496. doi: 10.1016/j.immuni.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu J, Chen ZJ. Innate immune sensing and signaling of cytosolic nucleic acids. Annu. Rev. Immunol. 2014;32:461–488. doi: 10.1146/annurev-immunol-032713-120156. [DOI] [PubMed] [Google Scholar]
- 43.Kato H, et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 44.Yoneyama M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 45.Kang DC, et al. Expression analysis and genomic characterization of human melanoma differentiation associated gene-5, mda-5: a novel type I interferon-responsive apoptosis-inducing gene. Oncogene. 2004;23:1789–1800. doi: 10.1038/sj.onc.1207300. [DOI] [PubMed] [Google Scholar]
- 46.Kowalinski E, et al. Structural basis for the activation of innate immune pattern-recognition receptor RIG-I by viral RNA. Cell. 2011;147:423–435. doi: 10.1016/j.cell.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 47.Vabret N, Blander JM. Sensing microbial RNA in the cytosol. Front. Immunol. 2013;4:468. doi: 10.3389/fimmu.2013.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanchez David RY, et al. Comparative analysis of viral RNA signatures on different RIG-I-like receptors. Elife. 2016;5:e11275. doi: 10.7554/eLife.11275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kato H, et al. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J. Exp. Med. 2008;205:1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu B, et al. Structural basis for dsRNA recognition, filament formation, and antiviral signal activation by MDA5. Cell. 2013;152:276–289. doi: 10.1016/j.cell.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 51.Liu HM, et al. The mitochondrial targeting chaperone 14-3-3epsilon regulates a RIG-I translocon that mediates membrane association and innate antiviral immunity. Cell Host Microbe. 2012;11:528–537. doi: 10.1016/j.chom.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin JP, Fan YK, Liu HM. The 14-3-3eta chaperone protein promotes antiviral innate immunity via facilitating MDA5 oligomerization and intracellular redistribution. PLoS Pathog. 2019;15:e1007582. doi: 10.1371/journal.ppat.1007582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goubau D, Deddouche S, Reis e Sousa C. Cytosolic sensing of viruses. Immunity. 2013;38:855–869. doi: 10.1016/j.immuni.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rothenfusser S, et al. The RNA helicase Lgp2 inhibits TLR-independent sensing of viral replication by retinoic acid-inducible gene-I. J. Immunol. 2005;175:5260–5268. doi: 10.4049/jimmunol.175.8.5260. [DOI] [PubMed] [Google Scholar]
- 55.Saito T, et al. Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proc. Natl Acad. Sci. USA. 2007;104:582–587. doi: 10.1073/pnas.0606699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quicke KM, Kim KY, Horvath CM, Suthar MS. RNA helicase LGP2 negatively regulates RIG-I signaling by preventing TRIM25-mediated caspase activation and recruitment domain ubiquitination. J. Interferon Cytokine Res. 2019;39:669–683. doi: 10.1089/jir.2019.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bruns AM, Leser GP, Lamb RA, Horvath CM. The innate immune sensor LGP2 activates antiviral signaling by regulating MDA5-RNA interaction and filament assembly. Mol. Cell. 2014;55:771–781. doi: 10.1016/j.molcel.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Venkataraman T, et al. Loss of DExD/H box RNA helicase LGP2 manifests disparate antiviral responses. J. Immunol. 2007;178:6444–6455. doi: 10.4049/jimmunol.178.10.6444. [DOI] [PubMed] [Google Scholar]
- 59.Satoh T, et al. LGP2 is a positive regulator of RIG-I- and MDA5-mediated antiviral responses. Proc. Natl Acad. Sci. USA. 2010;107:1512–1517. doi: 10.1073/pnas.0912986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gack MU, et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446:916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- 61.Oshiumi H, et al. The ubiquitin ligase Riplet is essential for RIG-I-dependent innate immune responses to RNA virus infection. Cell Host Microbe. 2010;8:496–509. doi: 10.1016/j.chom.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 62.Yan J, Li Q, Mao AP, Hu MM, Shu HB. TRIM4 modulates type I interferon induction and cellular antiviral response by targeting RIG-I for K63-linked ubiquitination. J. Mol. Cell Biol. 2014;6:154–163. doi: 10.1093/jmcb/mju005. [DOI] [PubMed] [Google Scholar]
- 63.Kuniyoshi K, et al. Pivotal role of RNA-binding E3 ubiquitin ligase MEX3C in RIG-I-mediated antiviral innate immunity. Proc. Natl Acad. Sci. USA. 2014;111:5646–5651. doi: 10.1073/pnas.1401674111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shen Y, et al. Riok3 inhibits the antiviral immune response by facilitating TRIM40-mediated RIG-I and MDA5 degradation. Cell Rep. 2021;35:109272. doi: 10.1016/j.celrep.2021.109272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rehwinkel J, Gack MU. RIG-I-like receptors: their regulation and roles in RNA sensing. Nat. Rev. Immunol. 2020;20:537–551. doi: 10.1038/s41577-020-0288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu MM, Liao CY, Yang Q, Xie XQ, Shu HB. Innate immunity to RNA virus is regulated by temporal and reversible sumoylation of RIG-I and MDA5. J. Exp. Med. 2017;214:973–989. doi: 10.1084/jem.20161015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gack MU, Nistal-Villan E, Inn KS, Garcia-Sastre A, Jung JU. Phosphorylation-mediated negative regulation of RIG-I antiviral activity. J. Virol. 2010;84:3220–3229. doi: 10.1128/JVI.02241-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seo J, et al. O-linked N-acetylglucosamine modification of mitochondrial antiviral signaling protein regulates antiviral signaling by modulating its activity. Front. Immunol. 2020;11:589259. doi: 10.3389/fimmu.2020.589259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lui PY, et al. PACT facilitates RNA-induced activation of MDA5 by promoting MDA5 oligomerization. J. Immunol. 2017;199:1846–1855. doi: 10.4049/jimmunol.1601493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kok KH, et al. The double-stranded RNA-binding protein PACT functions as a cellular activator of RIG-I to facilitate innate antiviral response. Cell Host Microbe. 2011;9:299–309. doi: 10.1016/j.chom.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 71.Lian H, et al. The zinc-finger protein ZCCHC3 binds RNA and facilitates viral RNA sensing and activation of the RIG-I-like receptors. Immunity. 2018;49:438–448.e435. doi: 10.1016/j.immuni.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 72.Oshiumi H, et al. DDX60 is involved in RIG-I-dependent and independent antiviral responses, and its function is attenuated by virus-induced EGFR activation. Cell Rep. 2015;11:1193–1207. doi: 10.1016/j.celrep.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 73.Zhou Z, et al. TRIM14 is a mitochondrial adaptor that facilitates retinoic acid-inducible gene-I-like receptor-mediated innate immune response. Proc. Natl Acad. Sci. USA. 2014;111:E245–E254. doi: 10.1073/pnas.1316941111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Acharya D, et al. Actin cytoskeleton remodeling primes RIG-I-like receptor activation. Cell. 2022;185:3588–3602.e3521. doi: 10.1016/j.cell.2022.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu L, Xiao N, Liu F, Ren H, Gu J. Inhibition of RIG-I and MDA5-dependent antiviral response by gC1qR at mitochondria. Proc. Natl Acad. Sci. USA. 2009;106:1530–1535. doi: 10.1073/pnas.0811029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paget M, et al. Stress granules are shock absorbers that prevent excessive innate immune responses to dsRNA. Mol. Cell. 2023;83:1180–1196.e1188. doi: 10.1016/j.molcel.2023.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hao, Y. et al. ZBP1: a powerful innate immune sensor and double-edged sword in host immunity. Int. J. Mol. Sci.23, 10.3390/ijms231810224 (2022). [DOI] [PMC free article] [PubMed]
- 78.Chan CP, Jin DY. Cytoplasmic RNA sensors and their interplay with RNA-binding partners in innate antiviral response: theme and variations. RNA. 2022;28:449–477. doi: 10.1261/rna.079016.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bartok E, Hartmann G. Immune sensing mechanisms that discriminate self from altered self and foreign nucleic acids. Immunity. 2020;53:54–77. doi: 10.1016/j.immuni.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Samuel CE. Adenosine deaminase acting on RNA (ADAR1), a suppressor of double-stranded RNA-triggered innate immune responses. J. Biol. Chem. 2019;294:1710–1720. doi: 10.1074/jbc.TM118.004166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ishii KJ, et al. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat. Immunol. 2006;7:40–48. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- 82.Takaoka A, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 83.Chiu YH, Macmillan JB, Chen ZJ. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell. 2009;138:576–591. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hornung V, Hartmann R, Ablasser A, Hopfner KP. OAS proteins and cGAS: unifying concepts in sensing and responding to cytosolic nucleic acids. Nat. Rev. Immunol. 2014;14:521–528. doi: 10.1038/nri3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gentili M, et al. The N-terminal domain of cGAS determines preferential association with centromeric DNA and innate immune activation in the nucleus. Cell Rep. 2019;26:2377–2393.e2313. doi: 10.1016/j.celrep.2019.01.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Du M, Chen ZJ. DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science. 2018;361:704–709. doi: 10.1126/science.aat1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou W, Mohr L, Maciejowski J, Kranzusch PJ. cGAS phase separation inhibits TREX1-mediated DNA degradation and enhances cytosolic DNA sensing. Mol. Cell. 2021;81:739–755.e737. doi: 10.1016/j.molcel.2021.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Luecke S, et al. cGAS is activated by DNA in a length-dependent manner. EMBO Rep. 2017;18:1707–1715. doi: 10.15252/embr.201744017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xie W, et al. Human cGAS catalytic domain has an additional DNA-binding interface that enhances enzymatic activity and liquid-phase condensation. Proc. Natl Acad. Sci. USA. 2019;116:11946–11955. doi: 10.1073/pnas.1905013116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Siddiqui MA, Yamashita M. Toll-like receptor (TLR) signaling enables cyclic GMP-AMP synthase (cGAS) sensing of HIV-1 infection in macrophages. mBio. 2021;12:e0281721. doi: 10.1128/mBio.02817-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yoh SM, et al. Recognition of HIV-1 capsid by PQBP1 licenses an innate immune sensing of nascent HIV-1 DNA. Mol. Cell. 2022;82:2871–2884.e2876. doi: 10.1016/j.molcel.2022.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Unterholzner L, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morrone SR, et al. Cooperative assembly of IFI16 filaments on dsDNA provides insights into host defense strategy. Proc. Natl Acad. Sci. USA. 2014;111:E62–E71. doi: 10.1073/pnas.1313577111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jiang Z, et al. IFI16 directly senses viral RNA and enhances RIG-I transcription and activation to restrict influenza virus infection. Nat. Microbiol. 2021;6:932–945. doi: 10.1038/s41564-021-00907-x. [DOI] [PubMed] [Google Scholar]
- 95.Sun H, et al. A nuclear export signal is required for cGAS to sense cytosolic DNA. Cell Rep. 2021;34:108586. doi: 10.1016/j.celrep.2020.108586. [DOI] [PubMed] [Google Scholar]
- 96.Almine JF, et al. IFI16 and cGAS cooperate in the activation of STING during DNA sensing in human keratinocytes. Nat. Commun. 2017;8:14392. doi: 10.1038/ncomms14392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Warga E, Anderson J, Tucker M, Harris E, Elmer J. Transcriptomic analysis of the innate immune response to in vitro transfection of plasmid DNA. Mol. Ther. Nucleic Acids. 2023;31:43–56. doi: 10.1016/j.omtn.2022.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yum, S., Li, M., Fang, Y. & Chen, Z. J. TBK1 recruitment to STING activates both IRF3 and NF-kappaB that mediate immune defense against tumors and viral infections. Proc. Natl Acad. Sci. USA118, 10.1073/pnas.2100225118 (2021). [DOI] [PMC free article] [PubMed]
- 99.Lee MN, et al. Identification of regulators of the innate immune response to cytosolic DNA and retroviral infection by an integrative approach. Nat. Immunol. 2013;14:179–185. doi: 10.1038/ni.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hertzog J, Rehwinkel J. Regulation and inhibition of the DNA sensor cGAS. EMBO Rep. 2020;21:e51345. doi: 10.15252/embr.202051345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gao D, et al. Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases. Proc. Natl Acad. Sci. USA. 2015;112:E5699–E5705. doi: 10.1073/pnas.1516465112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pokatayev V, et al. RNase H2 catalytic core Aicardi-Goutieres syndrome-related mutant invokes cGAS-STING innate immune-sensing pathway in mice. J. Exp. Med. 2016;213:329–336. doi: 10.1084/jem.20151464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schumann, T. et al. Deficiency for SAMHD1 activates MDA5 in a cGAS/STING-dependent manner. J. Exp. Med.220, 10.1084/jem.20220829 (2023). [DOI] [PMC free article] [PubMed]
- 104.Deng Y, Wang Y, Li L, Miao EA, Liu P. Post-translational modifications of proteins in cytosolic nucleic acid sensing signaling pathways. Front. Immunol. 2022;13:898724. doi: 10.3389/fimmu.2022.898724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen M, et al. TRIM14 inhibits cGAS degradation mediated by selective autophagy receptor p62 to promote innate immune responses. Mol. Cell. 2016;64:105–119. doi: 10.1016/j.molcel.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 106.Yang X, et al. MARCH8 attenuates cGAS-mediated innate immune responses through ubiquitylation. Sci. Signal. 2022;15:eabk3067. doi: 10.1126/scisignal.abk3067. [DOI] [PubMed] [Google Scholar]
- 107.Cai X, et al. Opposing effects of deubiquitinase OTUD3 in innate immunity against RNA and DNA viruses. Cell Rep. 2022;39:110920. doi: 10.1016/j.celrep.2022.110920. [DOI] [PubMed] [Google Scholar]
- 108.Song ZM, et al. KAT5 acetylates cGAS to promote innate immune response to DNA virus. Proc. Natl Acad. Sci. USA. 2020;117:21568–21575. doi: 10.1073/pnas.1922330117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dai J, et al. Acetylation blocks cGAS activity and inhibits self-DNA-induced autoimmunity. Cell. 2019;176:1447–1460.e1414. doi: 10.1016/j.cell.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hu MM, et al. Sumoylation promotes the stability of the DNA sensor cGAS and the adaptor STING to regulate the kinetics of response to DNA virus. Immunity. 2016;45:555–569. doi: 10.1016/j.immuni.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 111.Cui Y, et al. SENP7 potentiates cGAS activation by relieving SUMO-mediated inhibition of cytosolic DNA sensing. PLoS Pathog. 2017;13:e1006156. doi: 10.1371/journal.ppat.1006156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Seo GJ, et al. Akt kinase-mediated checkpoint of cGAS DNA sensing pathway. Cell Rep. 2015;13:440–449. doi: 10.1016/j.celrep.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun X, et al. DNA-PK deficiency potentiates cGAS-mediated antiviral innate immunity. Nat. Commun. 2020;11:6182. doi: 10.1038/s41467-020-19941-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang, X. et al. Aurora A kinase inhibition compromises its antitumor efficacy by elevating PD-L1 expression. J. Clin. Invest.133, 10.1172/JCI161929 (2023). [DOI] [PMC free article] [PubMed]
- 115.Wang, Y. et al. The role of O-GlcNAcylation in innate immunity and inflammation. J. Mol. Cell Biol.14, 10.1093/jmcb/mjac065 (2023). [DOI] [PMC free article] [PubMed]
- 116.Hu J, et al. Hexosamine biosynthetic pathway promotes the antiviral activity of SAMHD1 by enhancing O-GlcNAc transferase-mediated protein O-GlcNAcylation. Theranostics. 2021;11:805–823. doi: 10.7150/thno.50230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li T, Diner BA, Chen J, Cristea IM. Acetylation modulates cellular distribution and DNA sensing ability of interferon-inducible protein IFI16. Proc. Natl Acad. Sci. USA. 2012;109:10558–10563. doi: 10.1073/pnas.1203447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Baker PJ, et al. Posttranslational modification as a critical determinant of cytoplasmic innate immune recognition. Physiol. Rev. 2017;97:1165–1209. doi: 10.1152/physrev.00026.2016. [DOI] [PubMed] [Google Scholar]
- 119.Li D, et al. IFI16 isoforms with cytoplasmic and nuclear locations play differential roles in recognizing invaded DNA viruses. J. Immunol. 2021;207:2699–2709. doi: 10.4049/jimmunol.2100398. [DOI] [PubMed] [Google Scholar]
- 120.Zhao M, et al. The stress granule protein G3BP1 promotes pre-condensation of cGAS to allow rapid responses to DNA. EMBO Rep. 2022;23:e53166. doi: 10.15252/embr.202153166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang J, et al. ZYG11B potentiates the antiviral innate immune response by enhancing cGAS-DNA binding and condensation. Cell Rep. 2023;42:112278. doi: 10.1016/j.celrep.2023.112278. [DOI] [PubMed] [Google Scholar]
- 122.Tao X, et al. Ku proteins promote DNA binding and condensation of cyclic GMP-AMP synthase. Cell Rep. 2022;40:111310. doi: 10.1016/j.celrep.2022.111310. [DOI] [PubMed] [Google Scholar]
- 123.Lian H, et al. ZCCHC3 is a co-sensor of cGAS for dsDNA recognition in innate immune response. Nat. Commun. 2018;9:3349. doi: 10.1038/s41467-018-05559-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Singh RS, et al. DDX41 is required for cGAS-STING activation against DNA virus infection. Cell Rep. 2022;39:110856. doi: 10.1016/j.celrep.2022.110856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Saitoh T, et al. Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proc. Natl Acad. Sci. USA. 2009;106:20842–20846. doi: 10.1073/pnas.0911267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lv, T. et al. Targeting of GSDMD sensitizes HCC to anti-PD-1 by activating cGAS pathway and downregulating PD-L1 expression. J. Immunother. Cancer10, 10.1136/jitc-2022-004763 (2022). [DOI] [PMC free article] [PubMed]
- 127.Ghosh A, et al. Oligoadenylate-synthetase-family protein OASL inhibits activity of the DNA sensor cGAS during DNA virus infection to limit interferon production. Immunity. 2019;50:51–63.e55. doi: 10.1016/j.immuni.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liao CY, Lei CQ, Shu HB. PCBP1 modulates the innate immune response by facilitating the binding of cGAS to DNA. Cell Mol. Immunol. 2021;18:2334–2343. doi: 10.1038/s41423-020-0462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gu H, et al. PCBP2 maintains antiviral signaling homeostasis by regulating cGAS enzymatic activity via antagonizing its condensation. Nat. Commun. 2022;13:1564. doi: 10.1038/s41467-022-29266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Orzalli MH, et al. cGAS-mediated stabilization of IFI16 promotes innate signaling during herpes simplex virus infection. Proc. Natl Acad. Sci. USA. 2015;112:E1773–E1781. doi: 10.1073/pnas.1424637112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jonsson KL, et al. IFI16 is required for DNA sensing in human macrophages by promoting production and function of cGAMP. Nat. Commun. 2017;8:14391. doi: 10.1038/ncomms14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li D, et al. STING-mediated IFI16 degradation negatively controls type I interferon production. Cell Rep. 2019;29:1249–1260.e1244. doi: 10.1016/j.celrep.2019.09.069. [DOI] [PubMed] [Google Scholar]
- 133.Luteijn RD, et al. SLC19A1 transports immunoreactive cyclic dinucleotides. Nature. 2019;573:434–438. doi: 10.1038/s41586-019-1553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cordova AF, Ritchie C, Bohnert V, Li L. Human SLC46A2 IS the Dominant cGAMP importer in extracellular cGAMP-sensing macrophages and monocytes. ACS Cent. Sci. 2021;7:1073–1088. doi: 10.1021/acscentsci.1c00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou C, et al. Transfer of cGAMP into bystander cells via LRRC8 volume-regulated anion channels augments STING-mediated interferon responses and anti-viral immunity. Immunity. 2020;52:767–781.e766. doi: 10.1016/j.immuni.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 136.Wei X, et al. LL-37 transports immunoreactive cGAMP to activate STING signaling and enhance interferon-mediated host antiviral immunity. Cell Rep. 2022;39:110880. doi: 10.1016/j.celrep.2022.110880. [DOI] [PubMed] [Google Scholar]
- 137.Zhou Y, et al. Blockade of the phagocytic receptor MerTK on tumor-associated macrophages enhances P2X7R-dependent STING activation by tumor-derived cGAMP. Immunity. 2020;52:357–373.e359. doi: 10.1016/j.immuni.2020.01.014. [DOI] [PubMed] [Google Scholar]
- 138.Pepin, G. et al. Connexin-dependent transfer of cGAMP to phagocytes modulates antiviral responses. mBio11, 10.1128/mBio.03187-19 (2020). [DOI] [PMC free article] [PubMed]
- 139.Maltbaek JH, Cambier S, Snyder JM, Stetson DB. ABCC1 transporter exports the immunostimulatory cyclic dinucleotide cGAMP. Immunity. 2022;55:1799–1812.e1794. doi: 10.1016/j.immuni.2022.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Carozza JA, et al. ENPP1’s regulation of extracellular cGAMP is a ubiquitous mechanism of attenuating STING signaling. Proc. Natl Acad. Sci. USA. 2022;119:e2119189119. doi: 10.1073/pnas.2119189119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zahid A, Ismail H, Li B, Jin T. Molecular and structural basis of DNA sensors in antiviral innate immunity. Front Immunol. 2020;11:613039. doi: 10.3389/fimmu.2020.613039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fuertes MB, Woo SR, Burnett B, Fu YX, Gajewski TF. Type I interferon response and innate immune sensing of cancer. Trends Immunol. 2013;34:67–73. doi: 10.1016/j.it.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Suter MA, et al. cGAS-STING cytosolic DNA sensing pathway is suppressed by JAK2-STAT3 in tumor cells. Sci. Rep. 2021;11:7243. doi: 10.1038/s41598-021-86644-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wolff JA, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 145.Miao L, Zhang Y, Huang L. mRNA vaccine for cancer immunotherapy. Mol. Cancer. 2021;20:41. doi: 10.1186/s12943-021-01335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ishii KJ, et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451:725–729. doi: 10.1038/nature06537. [DOI] [PubMed] [Google Scholar]
- 147.Coban C, et al. Novel strategies to improve DNA vaccine immunogenicity. Curr. Gene Ther. 2011;11:479–484. doi: 10.2174/156652311798192815. [DOI] [PubMed] [Google Scholar]
- 148.Kobiyama K, Ishii KJ. Making innate sense of mRNA vaccine adjuvanticity. Nat. Immunol. 2022;23:474–476. doi: 10.1038/s41590-022-01168-4. [DOI] [PubMed] [Google Scholar]
- 149.Mu X, Hur S. Immunogenicity of In Vitro-Transcribed RNA. Acc. Chem. Res. 2021;54:4012–4023. doi: 10.1021/acs.accounts.1c00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sprent, J. & King, C. COVID-19 vaccine side effects: The positives about feeling bad. Sci Immunol6, 10.1126/sciimmunol.abj9256 (2021). [DOI] [PMC free article] [PubMed]
- 151.Miura N, Shaheen SM, Akita H, Nakamura T, Harashima H. A KALA-modified lipid nanoparticle containing CpG-free plasmid DNA as a potential DNA vaccine carrier for antigen presentation and as an immune-stimulative adjuvant. Nucleic Acids Res. 2015;43:1317–1331. doi: 10.1093/nar/gkv008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li A, et al. Activating cGAS-STING pathway for the optimal effect of cancer immunotherapy. J. Hematol. Oncol. 2019;12:35. doi: 10.1186/s13045-019-0721-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jeremiah, N. et al. RELA tunes innate-like interferon I/III responses in human T cells. J. Exp. Med.220, 10.1084/jem.20220666 (2023). [DOI] [PMC free article] [PubMed]
- 154.Marcus A, et al. Tumor-derived cGAMP triggers a STING-mediated interferon response in non-tumor cells to activate the NK cell response. Immunity. 2018;49:754–763 .e754. doi: 10.1016/j.immuni.2018.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schadt L, et al. Cancer-cell-intrinsic cGAS expression mediates tumor immunogenicity. Cell Rep. 2019;29:1236–1248.e1237. doi: 10.1016/j.celrep.2019.09.065. [DOI] [PubMed] [Google Scholar]
- 156.Feng X, et al. ATR inhibition potentiates ionizing radiation-induced interferon response via cytosolic nucleic acid-sensing pathways. EMBO J. 2020;39:e104036. doi: 10.15252/embj.2019104036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Du SS, et al. Radiation therapy promotes hepatocellular carcinoma immune cloaking via PD-L1 upregulation induced by cGAS-STING activation. Int J. Radiat. Oncol. Biol. Phys. 2022;112:1243–1255. doi: 10.1016/j.ijrobp.2021.12.162. [DOI] [PubMed] [Google Scholar]
- 158.Jesenko, T. et al. Radiation induced upregulation of DNA sensing pathways is cell-type dependent and can mediate the off-target effects. Cancers (Basel)12, 10.3390/cancers12113365 (2020). [DOI] [PMC free article] [PubMed]
- 159.Tian Z, Zeng Y, Peng Y, Liu J, Wu F. Cancer immunotherapy strategies that target the cGAS-STING pathway. Front. Immunol. 2022;13:996663. doi: 10.3389/fimmu.2022.996663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jiang Y, et al. Exploiting RIG-I-like receptor pathway for cancer immunotherapy. J. Hematol. Oncol. 2023;16:8. doi: 10.1186/s13045-023-01405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Skopelja-Gardner S, An J, Elkon KB. Role of the cGAS-STING pathway in systemic and organ-specific diseases. Nat. Rev. Nephrol. 2022;18:558–572. doi: 10.1038/s41581-022-00589-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fu J, et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci. Transl. Med. 2015;7:283ra252. doi: 10.1126/scitranslmed.aaa4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Heidegger S, et al. RIG-I activating immunostimulatory RNA boosts the efficacy of anticancer vaccines and synergizes with immune checkpoint blockade. EBioMedicine. 2019;41:146–155. doi: 10.1016/j.ebiom.2019.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu J, et al. PRMT1 mediated methylation of cGAS suppresses anti-tumor immunity. Nat. Commun. 2023;14:2806. doi: 10.1038/s41467-023-38443-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ma, D. et al. Arginine methyltransferase PRMT5 negatively regulates cGAS-mediated antiviral immune response. Sci. Adv.7, 10.1126/sciadv.abc1834 (2021). [DOI] [PMC free article] [PubMed]
- 166.Boehmer, D. F. R. et al. OAS1/RNase L executes RIG-I ligand-dependent tumor cell apoptosis. Sci. Immunol.6, 10.1126/sciimmunol.abe2550 (2021). [DOI] [PubMed]
- 167.Tudor D, et al. TLR9 pathway is involved in adjuvant effects of plasmid DNA-based vaccines. Vaccine. 2005;23:1258–1264. doi: 10.1016/j.vaccine.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 168.Moseman EA, et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J. Immunol. 2004;173:4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 169.Baghban, R., Ghasemian, A. & Mahmoodi, S. Nucleic acid-based vaccine platforms against the coronavirus disease 19 COVID-19. Arch. Microbiol.205, 150 (2023). [DOI] [PMC free article] [PubMed]
- 170.Stevenson EM, et al. SARS CoV-2 mRNA vaccination exposes latent HIV to Nef-specific CD8(+) T-cells. Nat. Commun. 2022;13:4888. doi: 10.1038/s41467-022-32376-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hemann EA, et al. A Small Molecule RIG-I Agonist Serves as an Adjuvant to Induce Broad Multifaceted Influenza Virus Vaccine Immunity. J. Immunol. 2023;210:1247–1256. doi: 10.4049/jimmunol.2300026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Apostolico JS, et al. Poly(I:C) potentiates T cell immunity to a dendritic cell targeted HIV-multiepitope vaccine. Front. Immunol. 2019;10:843. doi: 10.3389/fimmu.2019.00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rodell CB, et al. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat. Biomed. Eng. 2018;2:578–588. doi: 10.1038/s41551-018-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhou B, Li C, Yang Y, Wang Z. RIG-I promotes cell death in hepatocellular carcinoma by inducing M1 polarization of perineal macrophages through the RIG-I/MAVS/NF-kappaB pathway. Onco Targets Ther. 2020;13:8783–8794. doi: 10.2147/OTT.S258450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yang R, Yu S, Xu T, Zhang J, Wu S. Emerging role of RNA sensors in tumor microenvironment and immunotherapy. J. Hematol. Oncol. 2022;15:43. doi: 10.1186/s13045-022-01261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Crowl JT, Gray EE, Pestal K, Volkman HE, Stetson DB. Intracellular nucleic acid detection in autoimmunity. Annu. Rev. Immunol. 2017;35:313–336. doi: 10.1146/annurev-immunol-051116-052331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jiang, X. et al. Intrinsic RIG-I restrains STAT5 activation to modulate antitumor activity of CD8+ T cells. J. Clin. Invest.133, 10.1172/JCI160790 (2023). [DOI] [PMC free article] [PubMed]
- 178.Zheng, W. et al. How the innate immune DNA sensing cGAS-STING pathway is involved in apoptosis. Int. J. Mol. Sci.24, 10.3390/ijms24033029 (2023). [DOI] [PMC free article] [PubMed]
- 179.Kuhl N, et al. STING agonism turns human T cells into interferon-producing cells but impedes their functionality. EMBO Rep. 2023;24:e55536. doi: 10.15252/embr.202255536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Murthy AMV, Robinson N, Kumar S. Crosstalk between cGAS-STING signaling and cell death. Cell Death Differ. 2020;27:2989–3003. doi: 10.1038/s41418-020-00624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gu C, et al. Transfected DNA is targeted by STING-mediated restriction. Biochem. Biophys. Res. Commun. 2021;549:207–213. doi: 10.1016/j.bbrc.2021.02.109. [DOI] [PubMed] [Google Scholar]
- 182.Burke JM, et al. RNase L activation in the cytoplasm induces aberrant processing of mRNAs in the nucleus. PLoS Pathog. 2022;18:e1010930. doi: 10.1371/journal.ppat.1010930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhang F, et al. Binding of double-stranded RNA to protein kinase PKR is required for dimerization and promotes critical autophosphorylation events in the activation loop. J. Biol. Chem. 2001;276:24946–24958. doi: 10.1074/jbc.M102108200. [DOI] [PubMed] [Google Scholar]
- 184.Fleith RC, et al. IFIT3 and IFIT2/3 promote IFIT1-mediated translation inhibition by enhancing binding to non-self RNA. Nucleic Acids Res. 2018;46:5269–5285. doi: 10.1093/nar/gky191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Fang E, et al. Advances in COVID-19 mRNA vaccine development. Signal Transduct. Target Ther. 2022;7:94. doi: 10.1038/s41392-022-00950-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kim SC, et al. Modifications of mRNA vaccine structural elements for improving mRNA stability and translation efficiency. Mol. Cell Toxicol. 2022;18:1–8. doi: 10.1007/s13273-021-00171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yang B, Jeang J, Yang A, Wu TC, Hung CF. DNA vaccine for cancer immunotherapy. Hum. Vaccin. Immunother. 2014;10:3153–3164. doi: 10.4161/21645515.2014.980686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lama L, et al. Development of human cGAS-specific small-molecule inhibitors for repression of dsDNA-triggered interferon expression. Nat. Commun. 2019;10:2261. doi: 10.1038/s41467-019-08620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhang, S., Zheng, R., Pan, Y. & Sun, H. Potential Therapeutic Value of the STING Inhibitors. Molecules28, 10.3390/molecules28073127 (2023). [DOI] [PMC free article] [PubMed]
- 190.Wiser C, Kim B, Vincent J, Ascano M. Small molecule inhibition of human cGAS reduces total cGAMP output and cytokine expression in cells. Sci. Rep. 2020;10:7604. doi: 10.1038/s41598-020-64348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Steinhagen F, et al. Suppressive oligodeoxynucleotides containing TTAGGG motifs inhibit cGAS activation in human monocytes. Eur. J. Immunol. 2018;48:605–611. doi: 10.1002/eji.201747338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Valentin R, et al. Sequence-dependent inhibition of cGAS and TLR9 DNA sensing by 2’-O-methyl gapmer oligonucleotides. Nucleic Acids Res. 2021;49:6082–6099. doi: 10.1093/nar/gkab451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yu, H., Bruneau, R. C., Brennan, G. & Rothenburg, S. Battle royale: innate recognition of poxviruses and viral immune evasion. Biomedicines9, 10.3390/biomedicines9070765 (2021). [DOI] [PMC free article] [PubMed]
- 194.Chan YK, Gack MU. Viral evasion of intracellular DNA and RNA sensing. Nat. Rev. Microbiol. 2016;14:360–373. doi: 10.1038/nrmicro.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.El-Jesr M, Teir M, Maluquer de Motes C. Vaccinia virus activation and antagonism of cytosolic DNA sensing. Front. Immunol. 2020;11:568412. doi: 10.3389/fimmu.2020.568412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Eaglesham JB, Pan Y, Kupper TS, Kranzusch PJ. Viral and metazoan poxins are cGAMP-specific nucleases that restrict cGAS-STING signalling. Nature. 2019;566:259–263. doi: 10.1038/s41586-019-0928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jaafar ZA, Kieft JS. Viral RNA structure-based strategies to manipulate translation. Nat. Rev. Microbiol. 2019;17:110–123. doi: 10.1038/s41579-018-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Koliopoulos MG, et al. Molecular mechanism of influenza A NS1-mediated TRIM25 recognition and inhibition. Nat. Commun. 2018;9:1820. doi: 10.1038/s41467-018-04214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Minnaert, A. K. et al. Vaccinia virus protein B18R: influence on mRNA immunogenicity and translation upon non-viral delivery in different ocular cell types. Pharmaceutics13, 10.3390/pharmaceutics13010074 (2021). [DOI] [PMC free article] [PubMed]
- 200.Wuebben C, Bartok E, Hartmann G. Innate sensing of mRNA vaccines. Curr. Opin. Immunol. 2022;79:102249. doi: 10.1016/j.coi.2022.102249. [DOI] [PubMed] [Google Scholar]
- 201.Devarkar SC, et al. Structural basis for m7G recognition and 2’-O-methyl discrimination in capped RNAs by the innate immune receptor RIG-I. Proc. Natl Acad. Sci. USA. 2016;113:596–601. doi: 10.1073/pnas.1515152113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wesselhoeft RA, Kowalski PS, Anderson DG. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat. Commun. 2018;9:2629. doi: 10.1038/s41467-018-05096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wesselhoeft RA, et al. RNA circularization diminishes immunogenicity and can extend translation duration in vivo. Mol. Cell. 2019;74:508–520.e504. doi: 10.1016/j.molcel.2019.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chen YG, et al. N6-methyladenosine modification controls circular RNA immunity. Mol. Cell. 2019;76:96–109.e109. doi: 10.1016/j.molcel.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Moradian H, Roch T, Anthofer L, Lendlein A, Gossen M. Chemical modification of uridine modulates mRNA-mediated proinflammatory and antiviral response in primary human macrophages. Mol. Ther. Nucleic Acids. 2022;27:854–869. doi: 10.1016/j.omtn.2022.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang Y, et al. mRNA vaccine: a potential therapeutic strategy. Mol. Cancer. 2021;20:33. doi: 10.1186/s12943-021-01311-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Mei, Y. & Wang, X. RNA modification in mRNA cancer vaccines. Clin. Exp. Med.23, 1917–1931 (2023). [DOI] [PMC free article] [PubMed]
- 208.Cha EB, Shin KK, Seo J, Oh DB. Antibody-secreting macrophages generated using CpG-free plasmid eliminate tumor cells through antibody-dependent cellular phagocytosis. BMB Rep. 2020;53:442–447. doi: 10.5483/BMBRep.2020.53.8.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sheridan C. First COVID-19 DNA vaccine approved, others in hot pursuit. Nat. Biotechnol. 2021;39:1479–1482. doi: 10.1038/d41587-021-00023-5. [DOI] [PubMed] [Google Scholar]
- 210.Tse SW, et al. mRNA-encoded, constitutively active STING(V155M) is a potent genetic adjuvant of antigen-specific CD8(+) T cell response. Mol. Ther. 2021;29:2227–2238. doi: 10.1016/j.ymthe.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chen Z, et al. An mRNA vaccine elicits STING-dependent antitumor immune responses. Acta Pharm. Sin. B. 2023;13:1274–1286. doi: 10.1016/j.apsb.2022.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chen J, et al. Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8(+) T cell response. Proc. Natl Acad. Sci. USA. 2022;119:e2207841119. doi: 10.1073/pnas.2207841119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wang Q, et al. STING agonism reprograms tumor-associated macrophages and overcomes resistance to PARP inhibition in BRCA1-deficient models of breast cancer. Nat. Commun. 2022;13:3022. doi: 10.1038/s41467-022-30568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Parayath NN, Stephan SB, Koehne AL, Nelson PS, Stephan MT. In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat. Commun. 2020;11:6080. doi: 10.1038/s41467-020-19486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Johnson LR, et al. The immunostimulatory RNA RN7SL1 enables CAR-T cells to enhance autonomous and endogenous immune function. Cell. 2021;184:4981–4995.e4914. doi: 10.1016/j.cell.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Anwar S, Ul Islam K, Azmi MI, Iqbal J. cGAS-STING-mediated sensing pathways in DNA and RNA virus infections: crosstalk with other sensing pathways. Arch. Virol. 2021;166:3255–3268. doi: 10.1007/s00705-021-05211-x. [DOI] [PubMed] [Google Scholar]
- 217.Linares-Fernandez S, Lacroix C, Exposito JY, Verrier B. Tailoring mRNA vaccine to balance innate/adaptive immune response. Trends Mol. Med. 2020;26:311–323. doi: 10.1016/j.molmed.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 218.Colombani T, Haudebourg T, Pitard B. 704/DNA vaccines leverage cytoplasmic DNA stimulation to promote anti-HIV neutralizing antibody production in mice and strong immune response against alpha-fetoprotein in non-human primates. Mol. Ther. Nucleic Acids. 2023;32:743–757. doi: 10.1016/j.omtn.2023.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]