Abstract
Accumulation of 14α-methylated sterols or Δ8-sterols in Ustilago maydis affected three aspects of the plasma membrane H+-ATPase. Proton transport was reduced in Δ8-sterol-accumulating samples, due to an altered H+/ATP stoichiometry. ATP hydrolytic activity was increased, but no direct correlation with the extent or type of abnormal sterol accumulated could be drawn. Finally, Western blot analysis with antibodies against yeast PMA1 revealed a second lighter band (99-kDa band) in all samples from abnormal-sterol-accumulating sporidia. The conclusions are that the 99-kDa band and a reduced stoichiometry are directly linked to the presence of abnormal sterols, while changes in hydrolytic activity are linked only indirectly.
H+-ATPase is one of the major proteins in plasma membranes of plants and fungi, and it is involved in many adaptive and regulatory processes. Many of the stress responses in fungi induce changes in polypeptide amount and/or activity of the pump. For example, in Saccharomyces cerevisiae, decreases in H+-ATPase polypeptide are observed in stress situations brought about by nitrogen starvation (3), heat shock (12), or ethanol (14), while increases are seen in Zygosaccharomyces rouxii upon addition of NaCl to the growth medium (21). On the other hand, the activity of the pump is influenced by the same stress situations and the metabolic state of the cell, often independently of such changes in the abundance of the polypeptide. Activations of pump activity by glucose (17), nitrogen starvation (3), decanoic acid (1), and ethanol (16) are some examples.
The action of some fungicides is thought to be based on changes of membrane properties brought about by intervention in the biosynthetic pathway of ergosterol. These interventions cause abnormal sterols to accumulate. To date, no report has dealt with the effects that abnormal-sterol accumulation may have on the fungal plasma membrane H+-ATPase, although the lipid composition of the plasma membrane is thought to be of great importance in membrane-bound enzyme activity. In another publication, we showed that it is useful to make a comparison between the effects of a fungicide on abnormal-sterol composition and those found in a mutant with a genetic block in the sterol biosynthesis pathway at the point where the fungicide is presumed to act (6). These comparisons showed that the fungicides produced effects, similar to stress adaptation processes, which were not found in the mutants (6).
In this paper, we report that accumulation of 14α-methylated or Δ8-sterols has effects on the polypeptide pattern, hydrolytic activity, proton transport, and H+/ATP stoichiometry of Ustilago maydis plasma membrane H+-ATPase in cells with abnormal 14α-methylated or Δ8-unsaturated sterols. We have again made comparisons between fungicide-treated sporidia and sterol-deficient mutants to identify the specific effects of abnormal-sterol accumulation.
Isolation of plasma membranes.
Wild-type U. maydis (IMI 103761) was cultured for 48 h in minimal medium (6) on a rotary shaker at 25°C. When appropriate, 2.5 μM triadimenol (triadimenol treatment [Tri-T]) or 0.1 μM fenpropimorph (fenpropimorph treatment [Fen-T]) was added as an ethanolic solution to cultures of the wild-type strain at the time of inoculation. Ethanol (0.025%, vol/vol), in the absence of fungicide (ethanol control [Et-C]), was also added to wild-type sporidia as a control, since this compound may provoke activation of the enzyme (16). Isogenic mutant strains A14 and P51 were kind gifts from J. A. Hargreaves (7, 8) and were cultured without additions, as was the above-mentioned parental strain as a control (WT). All cultures were in mid-log phase when harvested. Plasma membranes were isolated and purified by the aqueous two-phase polymer technique as previously described (5). The most relevant characteristics of the strains used in this study are summarized in Table 1. Details of the lipid compositions of the membranes were given in a previous article (6).
TABLE 1.
Relevant biological characteristics of U. maydis strains and plasma membrane sterol compositions
| Strain and treatment | Relevant genotype | Sterol biosynthetic step affected | Abnormal sterols in plasma membranes (%) |
|---|---|---|---|
| IMI 103761 | |||
| Et-C (0.025%, vol/vol) | Wild type | None | <3 |
| Tri-T (2.5 μM, in ethanol)a | Wild type | Sterol C-14α-demethylase | 27.5 |
| Fen-T (0.1 μM, in ethanol)a | Wild type | Sterol Δ8-Δ7-isomerase | 66.6 |
| WT (none) | Wild type | None | <3 |
| A14 (none) | erg11 | Sterol C-14α-demethylase | 36.0 |
| P51 (none) | erg2 | Sterol Δ8-Δ7-isomerase | 89.4 |
Final concentration, 0.025% (vol/vol).
Proton transport.
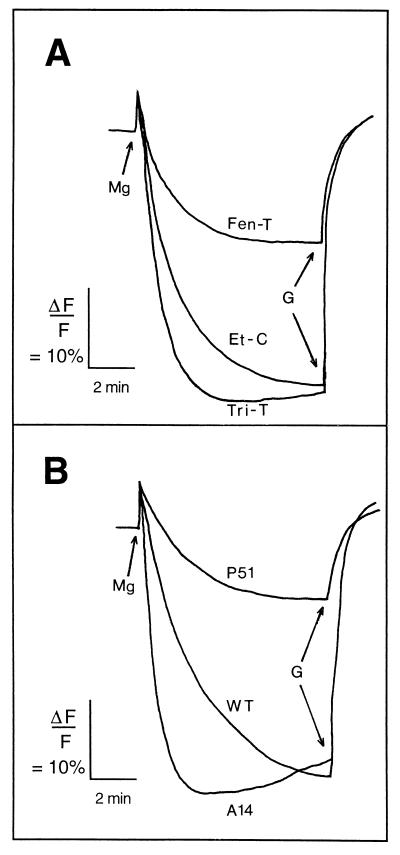
The generation of a Mg-ATP-dependent change in pH was assayed by monitoring the change in fluorescence emission of the fluorescent probe, 9-amino-6-chloro-2-methoxyacridine (ACMA) (Molecular Probes Ltd., Eugene, Oreg.), as described previously (4). The change in fluorescence emission was measured at 485 nm, with excitation at 415 nm, and recorded with a chart recorder. Vesicles were sealed, and the accumulated protons could be released by the addition of gramicidin D in all cases (Fig. 1). The rate of proton transport (VH+) in plasma membrane vesicles obtained from fungicide-treated sporidia depended strongly on the type of fungicide (Fig. 1A). The velocity of proton transport for Et-C sporidia was 185.5 ± 36.8 arbitrary units (AU) min−1 mg of protein−1 (mean ± standard error [SE]). Tri-T sporidia exhibited a slightly greater rate of proton pumping (231.4 ± 45.5 AU min−1 mg of protein−1), but Fen-T led to transport velocities which were only half those of Et-C organisms (98.6 ± 8.5 AU min−1 mg of protein−1). In WT control plasma membrane vesicles, proton pumping activity was 228.0 ± 22.1 AU min−1 mg of protein−1 (Fig. 1B), while in plasma membrane vesicles derived from the A14 mutant it was 2.5-fold greater (569.8 ± 75.2 AU min−1 mg of protein−1) and in those from the P51 mutant the rate was ca. 0.3 times that of the WT control (67.2 ± 7.8 AU min−1 mg of protein−1).
FIG. 1.
Proton transport across U. maydis plasma membrane vesicles measured by fluorescence quenching of the probe ACMA. The results are from typical experiments. (A) Fungicide-treated organisms; (B) sterol-deficient mutants. Gramicidin D (5-μg ml−1 final concentration) was added as indicated (G).
Vesicle sidedness and ATP hydrolysis.
Since rates of proton transport can be greatly influenced by the proportion of inside-out vesicles in the preparations, a series of latency tests on the hydrolytic activity of the plasma membrane H+-ATPase were done (Table 2). The medium for ATPase assay consisted of 0.33 M sucrose, 100 mM morpholineethanesulfonic acid (MES) adjusted to pH 6.5 with Tris, 1 mM sodium azide, 0.1 mM sodium molybdate, 50 mM potassium nitrate, 3 mM magnesium sulfate, 3.5 mM ATP (sodium salt), and 2 to 5 μg of membrane protein in a total volume of 240 μl. Assays were run for 10 min at 37°C. Under these conditions, the concentrations of Mg-ATP and free Mg2+ were 2.5 and 0.5 mM, respectively, as calculated with the program CHELATOR. The reaction was terminated by adding the stopping reagent for phosphate determination (11). Latency was determined in the presence and absence of 0.0125% (wt/vol) Triton X-100 in the reaction medium as described previously (5). Plasma membrane preparations from both the WT control and Et-C sporidia were a mixture of 50% inside-out (cytoplasmic-side-out) and 50% right-side-out vesicles. Strain A14 showed a remarkable increase in inside-out membranes. In contrast, Fen-T yielded preparations with nearly 1.5 times more right-side-out vesicles than Et-C. On the other hand, in plasma membranes from P51 and Tri-T sporidia the proportion of right-side-out vesicles did not differ from those in the WT control and Et-C organisms, respectively.
TABLE 2.
ATPase hydrolytic activities and proton transport rates in plasma membrane vesicles from U. maydis sporidiaa
| Strain and treatment | VH+ (AU min−1 mg of protein−1) | Hydrolytic activity (μmol of Pi min−1 mg−1) | Coupling index (AU [10−2] μmol of Pi−1) | Latency (%) |
|---|---|---|---|---|
| IMI 103761 | ||||
| Et-C | 391.6 ± 46.4 | 2.313 ± 0.059 | 2.92 ± 0.26 | 57.5 ± 6.1 |
| Tri-T | 872.8 ± 38.5 AC | 4.147 ± 0.475 AC | 2.70 ± 0.77 | 65.5 ± 1.7 C |
| Fen-T | 489.2 ± 78.5 D | 3.339 ± 0.295 A | 1.80 ± 0.31 AD | 72.9 ± 1.5 AD |
| WT | 457.8 ± 71.5 | 2.499 ± 0.061 | 3.00 ± 0.36 | 44.8 ± 8.3 |
| A14 (none) | 596.4 ± 65.0 | 1.761 ± 0.300 B | 4.20 ± 0.65 | 4.4 ± 4.4 B |
| P51 (none) | 171.2 ± 17.5 B | 2.893 ± 0.087 B | 0.71 ± 0.17 B | 53.3 ± 2.9 |
Data are means ± SEs of three independent experiments. Letters indicate significant differences as evaluated by the least-significant-difference test with respect to Et-C-treated sporidia (A), control WT sporidia (B), A14 (C), and P51 (D).
These differences in the proportions of transport-competent vesicles (i.e., inside-out) among strains were taken into account and used as correction factors for the observed VH+. After normalization (Table 2), the rates of proton transport showed that P51 strain still pumped protons 2.6 times more slowly than the WT control, while Tri-T organisms exhibited a ca. 2-fold greater rate of proton accumulation than Et-C organisms. However, rates in plasma membranes from Fen-T sporidia were not different from the values calculated for vesicles from Et-C sporidia (Table 2).
The activity of the pump measured as hydrolysis of ATP was seen to either increase or remain similar to values for controls in all cases (Table 2). Tri-T organisms displayed an increase in hydrolytic activity compared with Et-C organisms, as expected from the data on proton transport, but A14 showed no significant differences with respect to the WT control. In contrast, both Fen-T sporidia and P51 had greater ATP hydrolytic activities than the corresponding controls (i.e., 1.6 and 1.2 times above the levels measured for Et-C sporidia and the WT control, respectively), although the proton transport activities in these vesicles were lower. The latter figures suggested that the pump may be uncoupled in the presence of Δ8-sterols. Addition of fungicides to isolated plasma membrane vesicles did not have any effect on hydrolytic or proton pumping rates (data not shown).
H+/ATP coupling.
Aliquots of the assay medium of proton transport (100 μl) were taken before and after the reaction was started and were added to 140 μl of 15% (wt/vol) trichloroacetic acid, and the amount of phosphate released was determined. Coupling indices were calculated as the quotient of the observed proton pumping rate and the amount of phosphate released. These simultaneous measurements of phosphate release and proton transport gave values for coupling indices 1.6- and 4.2-fold lower for Fen-T and P51 than for Et-C and the WT, respectively, but no differences were observed when analyzing plasma membranes vesicles derived from 14α-methyl-sterol-accumulating sporidia (Table 2).
Glucose activation of the H+-ATPase.
The possibility that changes in sterol composition could lead to differences in the glucose activation of the pump was also considered. After 48 h of growth, cells were centrifuged and transferred to flasks containing fresh minimal medium with 2% glycerol as the sole carbon source for 1 h to assess the extent of glucose activation of the proton pump. However, plasma membranes obtained from glucose-fermenting and glucose-starved cells of the WT control showed no differences in ATPase activity or sensitivity to vanadate (1.418 ± 0.203 μmol of Pi min−1 mg−1 and 52.8% ± 1.6% inhibition by 10 μM vanadate for glucose-fermenting cells and 1.773 ± 0.010 μmol of Pi min−1 mg−1 and 50.5% ± 0.9% inhibition by 10 μM vanadate for glycerol-metabolizing sporidia [means ± SEs; n = 2]).
Western blot analysis of the plasma membrane H+-ATPase.
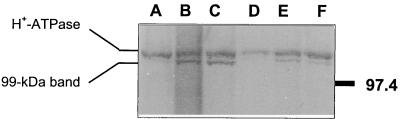
Plasma membrane proteins were separated electrophoretically in 8% polyacrylamide gels by using the buffer system of Laemmli (9), transferred to nitrocellulose, and probed with a polyclonal antibody raised against the yeast PMA1 gene product, diluted 5,000-fold. Blots were developed with alkaline phosphatase-linked secondary antibodies. All preparations showed a distinct band at ca. 104 kDa, the only present in preparations of plasma membranes from the WT control and Et-C sporidia (Fig. 2). Surprisingly, all treated and mutant strains showed not only this band but also a second one at ca. 99 kDa which was hardly visible in samples from the WT control and Et-C organisms, even in gels loaded with ca. 10 times more protein (data not shown). The facts that this band appears in plasma membranes from sporidia that accumulate abnormal sterols and that no degradation was observed in Coomassie blue-stained gels (data not shown) suggest that this 99-kDa band is not an artifact. On the other hand, a polyclonal antibody raised against the C-terminal domain of the yeast PMA1 gene product did not cross-react with any polypeptide (data not shown). Densitometric quantification of the bands showed no changes for the normal (104-kDa) band, with the exception of P51 with respect to the WT control (Table 3). On the other hand, the 99-kDa band was induced ca. threefold in both mutants and fungicide-treated sporidia in comparison to the WT control and Et-C organisms. No correlation was apparent between changes in hydrolytic activity or proton transport and amounts of H+-ATPase polypeptide or the 99-kDa band in samples from abnormal-sterol-accumulating organisms.
FIG. 2.
Western blot of plasma membrane fractions obtained from the different strains of U. maydis. Lanes: A, control WT sporidia; B, A14; C, P51; D, Et-C sporidia; E, Tri-T sporidia; F, Fen-T sporidia. Lanes were loaded with 5 μg of protein. At the right is the molecular mass (in kilodaltons) of the marker.
TABLE 3.
H+-ATPase and 99-kDa band quantities from U. maydis plasma membranesa
| Strain and treatment | Amt (AU mg of protein−1) of:
|
|
|---|---|---|
| H+-ATPase | 99-kDa protein | |
| IMI 103761 | ||
| Et-C | 16.5 ± 3.0 | 5.5 ± 1.3 |
| Tri-T | 20.5 ± 2.4 | 11.5 ± 0.8 A |
| Fen-T | 21.6 ± 2.2 | 9.8 ± 0.8 AC |
| WT | 16.9 ± 1.7 | 4.5 ± 0.2 |
| A14 (none) | 16.4 ± 2.1 | 11.9 ± 1.9 B |
| P51 (none) | 23.0 ± 2.3 B | 13.8 ± 0.6 B |
Data are means ± SEs for six experiments. Letters indicate significant differences evaluated by the least-significant-difference test (at a 95% confidence level) with respect to Et-C-treated sporidia (A), control WT sporidia (B), and P51 (C). No significant differences were found with respect to A14.
Conclusions.
Abnormal Δ8-sterols and C-14α-methylated sterols had contrary effects on the rate of proton pumping in U. maydis plasma membrane vesicles (Table 2). In the presence of Δ8-sterols, H+ translocation across the vesicles seemed to be inhibited. The effects that treatments, mutations, or different growth conditions may have on the sidedness of the vesicles have traditionally been disregarded. However, the way in which membranes reform after cell disruption depends on the physical characteristics of the bilayer, which can be affected by the sterol composition. Correction of the rates of H+ transport and comparison to the ATP hydrolytic activities suggested a change in the stoichiometry of the pump in plasma membranes of Δ8-sterol-accumulating organisms, while enhancement of hydrolytic activity was the cause for increased proton pumping in plasma membranes of 14α-methyl-sterol-accumulating organisms (Table 2). This view was supported by the fact that latency values decreased only in the case of A14, while in the other cases, the lack of changes or even increases in latency values indicated that abnormal-sterol accumulation did not prevent vesiculation of membranes upon cell breakage. Furthermore, these effects cannot be attributed to increased proton permeability of the lipid bilayer, since this parameter does not change appreciably in these vesicles (6). A change in the stoichiometry of the pump in Δ8-sterol-accumulating sporidia was confirmed by experiments in which ATP hydrolysis and VH+ were determined simultaneously (Table 2). Assuming a stoichiometry of one proton pumped per molecule of ATP consumed for enzymes of the WT control and Et-C sporidia (13, 18), P51 showed a stoichiometry of ca. 0.2 H+/ATP and Fen-T sporidia showed a stoichiometry of ca. 0.6 H+/ATP (Table 2). Similar values have been reported for the yeast plasma membrane H+-ATPase from cells grown in the absence of glucose (20). However, in this case, changes in glucose activation of the pump can be ruled out since no glucose activation has been observed. The mechanisms by which this uncoupling occurs remain obscure, although involvement of the C-terminal domains of the sarcoplasmic reticulum Ca2+-ATPase and S. cerevisiae plasma membrane H+-ATPase have been suggested in the case of these two P-type enzymes (2, 21). Also, the N-terminal domain has been associated with lipid-protein interactions (10). Therefore, the idea of a interaction of membrane sterols with the ATPase C or N terminus is a plausible explanation for the phenomenon observed in U. maydis plasma membrane vesicles, and it would be worth further investigation. On the other hand, sterols have access to nonannular binding sites of P-type ATPases (19).
The ATP hydrolytic activity did not show a recognizable pattern of variation in relation with the sterol composition. A small increase in the amount of H+-ATPase polypeptide may account for the increased hydrolytic activity observed in the P51 mutant and, less clearly, in the case of Fen-T sporidia. However, this explanation is not applicable to A14. Although in plasma membranes from abnormal-sterol-accumulating sporidia a second ATPase-like band was observed on Western blots (99-kDa band), no correlation between ATP hydrolytic activity increases and changes in amounts of the 99-kDa band was evident. The difference in molecular mass between H+-ATPase and the 99-kDa band is similar to the molecular mass of the C-terminal domain of the yeast H+-ATPase (15). However, a lack of cross-reactivity of U. maydis polypeptides with the yeast H+-ATPase C terminus-raised antibody prevented confirmation of the putative involvement of this domain.
The results presented here show that abnormal-sterol accumulation has direct and indirect effects on U. maydis plasma membrane H+-ATPase. Some of these effects are sterol type specific, such as the effect of Δ8-sterols on H+/ATP stoichiometry, while the induction of the 99-kDa band seems to occur in the presence of both types of abnormal sterols. Finally, the increases in ATP hydrolytic activity seem to be indirectly linked to the presence of abnormal sterols.
Acknowledgments
We thank R. Serrano and M. J. García and J. R. Murgia (IBMCP-UPVA, Valencia, Spain) for training A.H. in the Western blot technique and for the generous gift of the antibodies. We also thank T. J. M. Shoenmakers (Katholieke Universiteit Nijmegen, Nijmegen, The Netherlands) for providing the computer program CHELATOR.
A.H. was the recipient of a “Beca de Formación de Investigadores” from the Basque Government (Spain).
REFERENCES
- 1.Alexandre H, Mathieu B, Charpentier C. Alteration in membrane fluidity and lipid composition, and modulation of H+-ATPase activity in Saccharomyces cerevisiae caused by decanoic acid. Microbiology. 1996;142:469–475. doi: 10.1099/13500872-142-3-469. [DOI] [PubMed] [Google Scholar]
- 2.Andersen J P. Functional consequences of alterations to amino acids at the M5S5 boundary of the Ca2+-ATPase of sarcoplasmic reticulum—mutation Tyr763→Gly uncouples ATP hydrolysis from Ca2+ transport. J Biol Chem. 1995;270:908–914. doi: 10.1074/jbc.270.2.908. [DOI] [PubMed] [Google Scholar]
- 3.Benito B, Portillo F, Lagunas R. In vivo activation of the yeast plasma membrane ATPase during nitrogen starvation. Identification of the regulatory domain that controls activation. FEBS Lett. 1992;300:271–274. doi: 10.1016/0014-5793(92)80861-a. [DOI] [PubMed] [Google Scholar]
- 4.Coupland D, Cooke D T, James C S. Effects of 4-chloro-2-methylphenoxypropionate (an auxin analogue) on plasma membrane ATPase activity in herbicide-resistant and herbicide-susceptible biotypes of Stellaria media L. J Exp Bot. 1991;42:1065–1071. [Google Scholar]
- 5.Hernández A, Cooke D T, Clarkson D T. Lipid composition and proton transport in Penicillium cyclopium and Ustilago maydis plasma membrane vesicles isolated by two-phase partitioning. Biochim Biophys Acta. 1994;1195:103–109. doi: 10.1016/0005-2736(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 6.Hernández A, Cooke D T, Lewis M, Clarkson D T. Fungicides and sterol-deficient mutants of Ustilago maydis: plasma membrane physico-chemical characteristics do not explain growth inhibition. Microbiology. 1992;143:3165–3174. doi: 10.1099/00221287-143-10-3165. [DOI] [PubMed] [Google Scholar]
- 7.James C S, Burden R S, Loeffler R S T, Hargreaves J A. Isolation and characterisation of polyene-resistant mutants from the maize smut pathogen, Ustilago maydis, defective in ergosterol biosynthesis. J Gen Microbiol. 1992;138:1437–1443. [Google Scholar]
- 8.Keon J P R, Hargreaves J A. An Ustilago maydis mutant partially blocked in P45014DM activity is hypersensitive to azole fungicides. Fungal Genet Biol. 1996;20:84–88. doi: 10.1006/fgbi.1996.0014. [DOI] [PubMed] [Google Scholar]
- 9.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 10.Møller J V, Juul B, Le Maire M. Structural organization, ion transport, and energy transduction of P-type ATPases. Biochim Biophys Acta. 1996;1286:1–51. doi: 10.1016/0304-4157(95)00017-8. [DOI] [PubMed] [Google Scholar]
- 11.Onishi T, Gall R S, Mayer M L. An improved assay of inorganic phosphate in the presence of extra-labile phosphate compounds: application to the ATPase in the presence of phosphocreatine. Anal Biochem. 1975;69:261–267. doi: 10.1016/0003-2697(75)90585-0. [DOI] [PubMed] [Google Scholar]
- 12.Panaretou B, Piper P W. The plasma membrane of yeast acquires a novel heat-shock protein (hsp30) and displays a decline in proton-pumping ATPase levels in response to both heat shock and the entry to stationary phase. Eur J Biochem. 1992;206:635–640. doi: 10.1111/j.1432-1033.1992.tb16968.x. [DOI] [PubMed] [Google Scholar]
- 13.Perlin D S, San Francisco M J D, Slayman C W, Rosen B P. H+/ATP stoichiometry of proton pumps from Neurospora crassa and Escherichia coli. Arch Biochem Biophys. 1986;248:53–61. doi: 10.1016/0003-9861(86)90400-5. [DOI] [PubMed] [Google Scholar]
- 14.Piper P W, Talreja K, Panaretou B, Moradas-Ferreira P, Byrne K, Praekelt U M, Meacock P, Récnacp M, Boucherie H. Induction of major heat-shock proteins of Saccharomyces cerevisiae, including plasma membrane Hsp30, by ethanol levels above a critical threshold. Microbiology. 1994;140:3031–3038. doi: 10.1099/13500872-140-11-3031. [DOI] [PubMed] [Google Scholar]
- 15.Portillo F, Fernandez de Larrinoa I, Serrano R. Deletion analysis of yeast plasma membrane H+-ATPase and identification of a regulatory domain at the carboxyl-terminus. FEBS Lett. 1989;247:381–385. doi: 10.1016/0014-5793(89)81375-4. [DOI] [PubMed] [Google Scholar]
- 16.Rosa M F, Correia I S. In vivo activation by ethanol of plasma membrane ATPase of Saccharomyces cerevisiae. Appl Environ Microbiol. 1991;57:830–835. doi: 10.1128/aem.57.3.830-835.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serrano R. In vivo glucose activation of the yeast plasma membrane ATPase. FEBS Lett. 1983;156:11–14. doi: 10.1016/0014-5793(83)80237-3. [DOI] [PubMed] [Google Scholar]
- 18.Serrano R. Structure and function of proton translocating ATPase in plasma membranes of plants and fungi. Biochim Biophys Acta. 1988;947:1–28. doi: 10.1016/0304-4157(88)90017-2. [DOI] [PubMed] [Google Scholar]
- 19.Simmonds A C, East J M, Jones O T, Rooney E K, Lee A G. Annular and non-annular binding sites on the (Ca2+ + Mg2+)-ATPase. Biochim Biophys Acta. 1982;693:398–406. doi: 10.1016/0005-2736(82)90447-3. [DOI] [PubMed] [Google Scholar]
- 20.Venema K, Palmgren M G. Metabolic modulation of transport coupling ratio in yeast plasma membrane H+-ATPase. J Biol Chem. 1995;270:19659–19667. doi: 10.1074/jbc.270.33.19659. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe Y, Sanemitsu Y, Tamai Y. Expression of plasma membrane proton-ATPase gene in salt-tolerant yeast Zygosaccharomyces rouxii is induced by sodium chloride. FEMS Microbiol Lett. 1993;114:105–108. [Google Scholar]