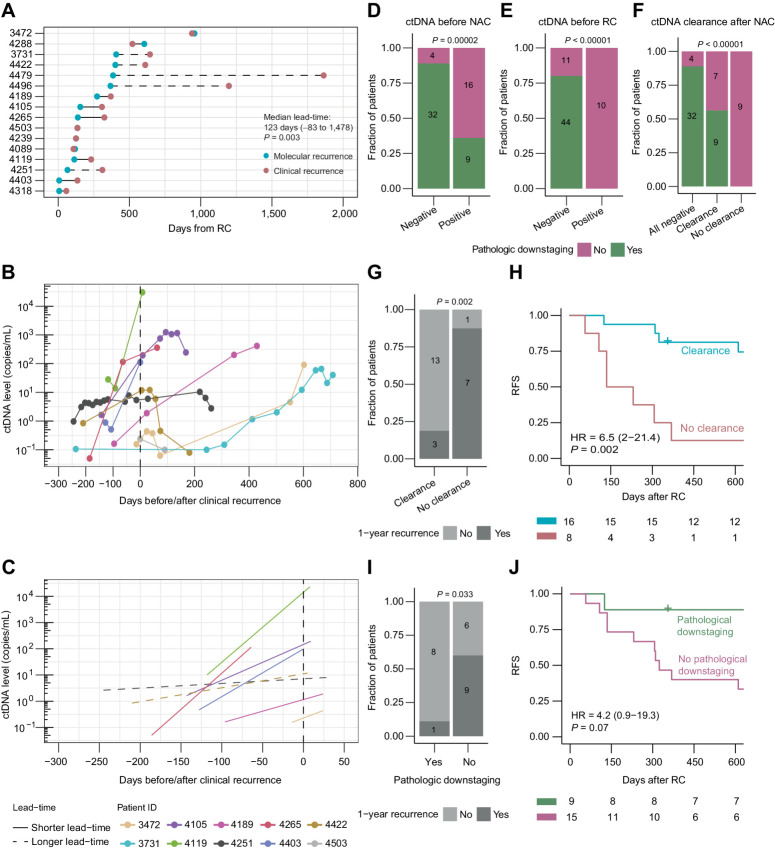
Figure 2.
ctDNA measurements for monitoring relapse and treatment response. A, Lead time in days between molecular recurrence (ctDNA positivity) and clinical recurrence (radiographic imaging positive). Statistical significance was calculated using paired Wilcoxon rank sum test. Longer lead-time was defined as >200 days between molecular and clinical recurrence. B, ctDNA levels at the time of clinical recurrence (radiographic imaging positive, time point zero) for patients having at least two plasma samples analyzed for ctDNA at the time of their clinical relapse. C, Linear regression lines of ctDNA levels at the time of clinical recurrence (radiographic imaging positive, time point zero) for patients having at least two plasma samples analyzed for ctDNA at the time of their clinical relapse. Longer lead-time was defined as > 200 days between molecular and clinical recurrence. D, Association between ctDNA status before NAC and pathologic downstaging. E, Association between ctDNA status before RC and pathologic downstaging. F, Association between ctDNA clearance after NAC and pathologic downstaging. G, Association between ctDNA clearance after NAC and recurrence status within 1 year after RC for patients being ctDNA-positive before NAC. H, Kaplan–Meier survival analysis of RFS and ctDNA clearance after NAC for patients being ctDNA-positive before NAC. I, Association between pathologic downstaging and recurrence status within 1 year after RC for patients being ctDNA-positive before NAC. J, Kaplan–Meier survival analysis of RFS and pathologic downstaging for patients being ctDNA-positive before NAC. HRs, associated 95% CIs, and P values are displayed on each Kaplan–Meier plot (Cox regression analysis). Significant statistical difference between categorical variables was determined using Fisher exact test.