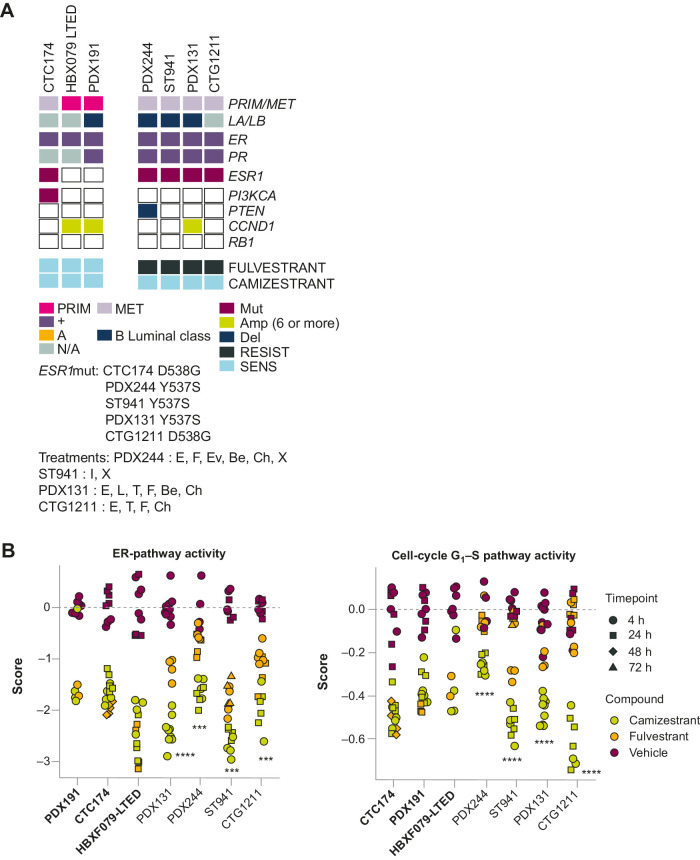
Figure 4.
Camizestrant has superior in vivo activity to fulvestrant in ESR1wt and ESR1m PDX models (2). A, Characteristics of ER+ breast cancer models used. B, Change in ER pathway gene activation after treatment, expressed as change in ER pathway gene score and in cell-cycle G1–S checkpoint genes. See Supplementary Methods for details. Statistical analysis comparing fulvestrant and camizestrant was done using one-way analysis of covariance (n ≥ 4 animals per group). Models shown in bold (x-axis) are fulvestrant sensitive; those in regular type are fulvestrant resistant. ***, P < 0.001; ****, P < 0.0001. Amp, amplification; CCND1, cyclin D1; Del, deletion; MET, metastasis; Mut, mutation; PIK3CA, phosphatidylinositol 3-kinase subunit α; PR, progesterone receptor; PRIM, primary; RESIST, resistant; RB1, retinoblastoma gene; SENS, sensitive. Patient treatment reported: Be, bevacizumab; Ch, chemotherapy; E, exemestane; Ev, everolimus; F, fulvestrant; I, investigational; L, letrozole, T, tamoxifen; X, radiotherapy.