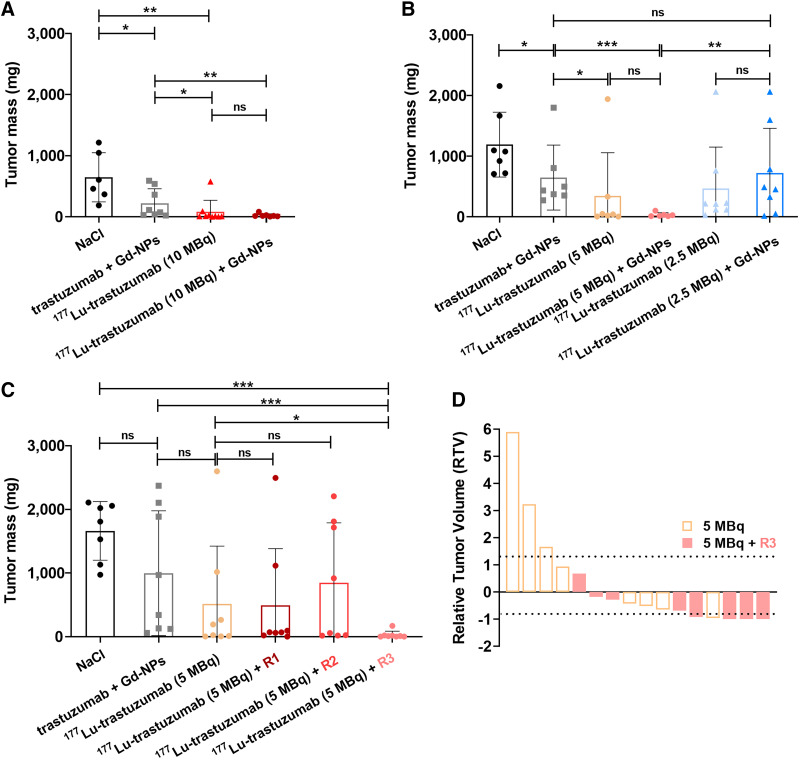
FIGURE 2.
Determination of therapeutic efficacy of [177Lu]Lu-DOTA-trastuzumab combined with Gd-NPs. (A and B) Tumor mass was determined in mice treated with maximum tolerated activity (10 MBq) of [177Lu]Lu-DOTA-trastuzumab followed or not (48 h later) by 10 mg of Gd-NPs (A) and low (2.5 MBq) or intermediate (5 MBq) activity of [177Lu]Lu-DOTA-trastuzumab followed or not (48 h later) by 10 mg of Gd-NPs (B). (C) Tumor mass in mice treated with 5 MBq of [177Lu]Lu-DOTA-trastuzumab regimen 1 (1 injection of 4 mg of Gd-NPs/d for 5 d starting 48 h after [177Lu]Lu-DOTA-trastuzumab injection), regimen 2 (2 injections of 2 mg of Gd-NPs/d for 5 d starting 48 h after [177Lu]Lu-DOTA-trastuzumab), or regimen 3 (2 injections of 5 mg of Gd-NPs per day at 24 and 72 h after [177Lu]Lu-DOTA-trastuzumab). (D) Relative tumor volume at treatment end. Results are mean ± SD. *P < 0.05. **P < 0.01. ***P < 0.001. ns = not significant (Mann–Whitney t test).