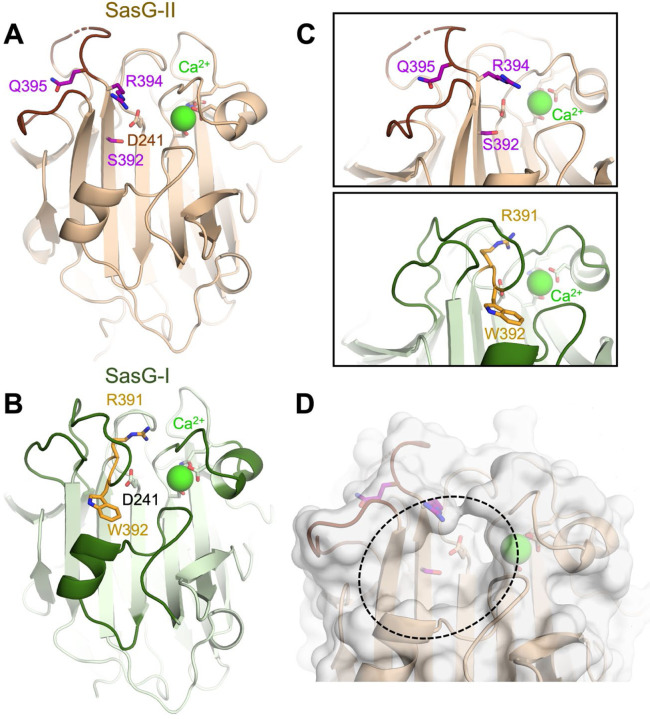
Figure 2. The SasG-II lectin contains a unique non-aromatic residue in the glycan binding pocket.
(A) Crystal structure of the SasG-II lectin showing the structural Ca2+ ion, the conserved central D241 residue that adopts an atypical trans conformation, and the side chains of S392, R394, and Q395 near the end of β17. (B) Comparative view of SasG-II in the same orientation. Residues R391 and W392 are analogous to R394 and Q395 in SasG-II; note the distinct positioning of corresponding residues W392 (SasG-II) and Q395 (SasG-I). (C) Close-up view of the region near the end of β17, rotated by approximately 45° from panels A and B. Note the sharp bend of the main chain near R391 in SasG-I that is not observed in SasG-II. (D) Surface view of SasG-II showing the putative binding pocket lacking an aromatic residue at its base.