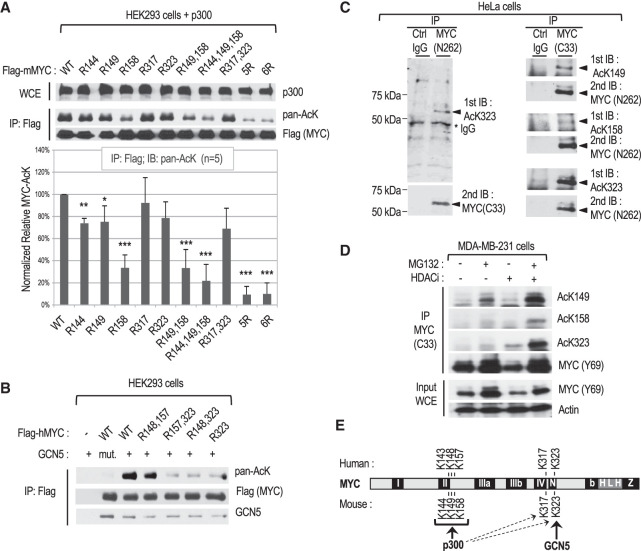
Figure 1.
Characterization of site-specific MYC acetylation in different cell lines. (A) Mapping of the main MYC lysine (K) residues acetylated by p300 in HEK293 cells transfected with p300 and FLAG-tagged mouse MYC or the indicated K-to-R substitution mutants. (Top) Representative Western blot with the indicated antibodies of whole-cell extracts (WCEs) and immunoprecipitated FLAG-mMYC. (Bottom) Chart showing the quantitation of acetylated MYC signals obtained with the pan-acetyl-lysine antibody from five independent experiments. (B) Mapping of the main human MYC lysine residue acetylated by GCN5 in transfected cells as above. (mut.) Acetylation-defective GCN5 HAT mutant. (C) Endogenous MYC in HeLa cervical cancer cells was immunoprecipitated with the indicated antibodies and analyzed by Western blot by immunoblotting (IB) with MYC AcK site-specific antibodies, followed by stripping and reprobing with a MYC antibody. (D) Analysis by IP and Western blot of endogenous site-specific acetylated MYC in MDA-MB-231 mammary cancer cells with or without MG132 and/or HDAC inhibitor (HDACi) treatment for 2 h before lysis. (E) Summary of MYC K site(s) preferentially acetylated by p300 and GCN5.