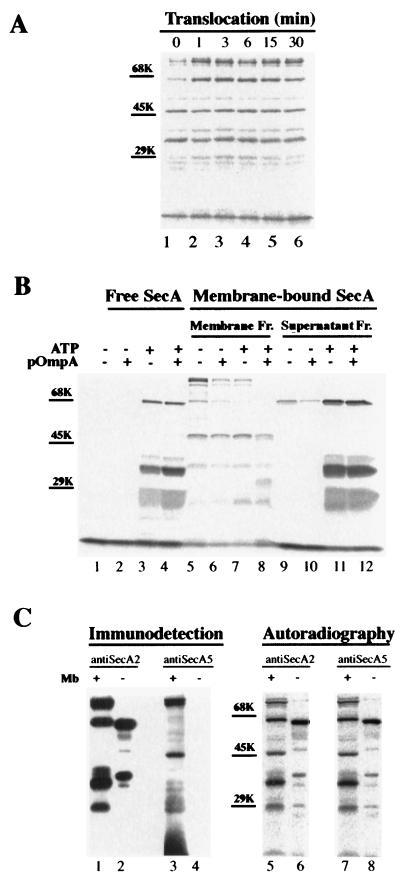
FIG. 4.
Characterization of the protease-resistant SecA domains. (A) Proteolysis of membrane-associated SecA at different translocation stages. After translocation and proteolysis as described in Materials and Methods, SecA fragments recovered from the membrane fraction were separated by SDS-PAGE and visualized by autoradiography. Positions of molecular-marker proteins are shown by bars. (B) Effects of ATP, proOmpA, and membrane on the formation of the proteolytic SecA fragments. 35S-labeled SecA was incubated at 37°C for 15 min under the conditions indicated. After the incubation, the reaction mixture was incubated with 1 mg of trypsin/ml on ice for 15 min. The SecA fragments generated in the absence of membrane were TCA precipitated and were analyzed by SDS-PAGE and autoradiography (lanes 1 to 4). The proteolytic SecA fragments generated in the presence of membranes were separated into membrane and supernatant fractions (Fr.), followed by SDS-PAGE and autoradiography (lanes 5 to 12). Positions for molecular-weight marker proteins are indicated. (C) Limited proteolysis of SecA in the absence of membranes (Mb). After incubation in translocation mixture minus membranes, 35S-labeled SecA was incubated with 60 μg of trypsin/ml on ice for 15 min and precipitated by 8% TCA. The pelleted 35S-labeled SecA fragments were separated by SDS-PAGE and visualized by autoradiography (lanes 6 and 8) and by immunodetection with anti-SecA2 (lane 2) and anti-SecA5 (lane 4). SecA fragments from trypsinized (1 mg/ml; 15 min) membranes were analyzed for comparison (lanes 1, 3, 5, and 7).