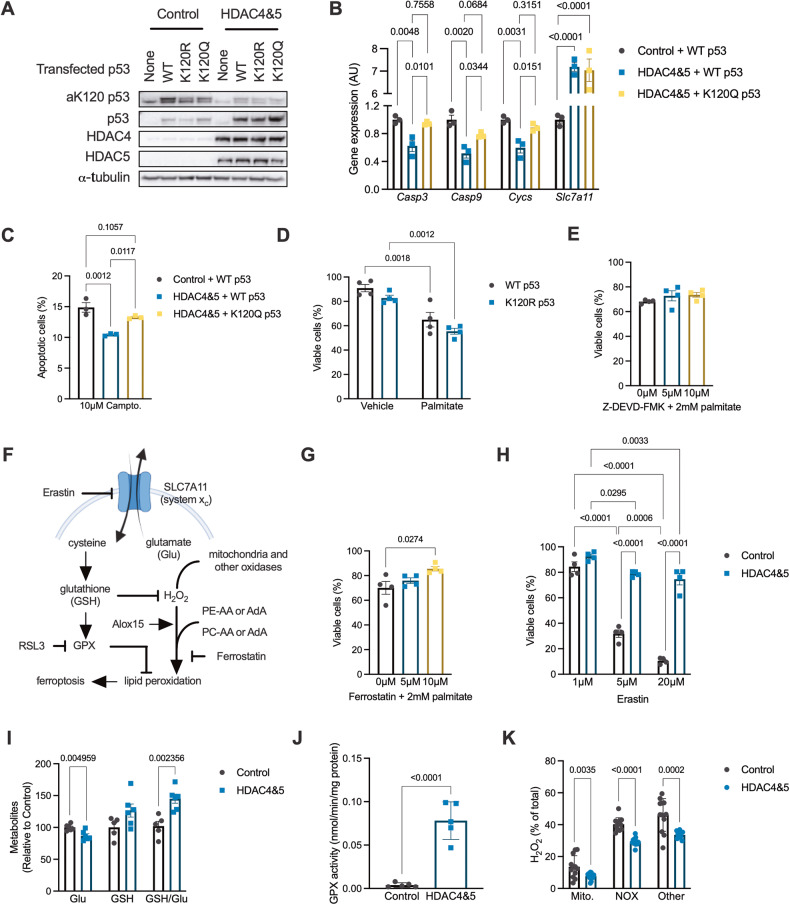
Fig. 5. HDAC4 and 5 inhibit apoptosis by reducing p53 K120 acetylation and reduces multiple metabolic inputs to the ferroptosis pathway.
A Acetylated p53 at lysine 120 (aK120 p53) in Control or HDAC4 and 5 overexpressing C2C12 myoblasts also expressing wild type (WT), K120R (loss-of-function) or K120Q (gain-of-function) p53. B Expression of caspase 3 (Casp3; One-way ANOVA p = 0.0040 ×2 = 0.8408), caspase 9 (Casp9; One-way ANOVA p = 0.0025 ×2 = 0.8650), cytochrome c (Cycs; One-way ANOVA p = 0.0033 ×2 = 0.8518) and the cystine/glutamate antiporter xCT (Slc7a11; One-way ANOVA p < 0.0001 ×2 = 0.9769) in Control or HDAC4 and 5 overexpressing C2C12 myoblasts also expressing WT or K120Q p53 (Tukey’s multiple comparisons shown). C Percentage of apoptotic cells following exposure to camptothecin in Control or HDAC4 and 5 overexpressing C2C12 myoblasts also expressing WT or K120Q p53 (One-way ANOVA p = 0.0014 ×2 = 0.8875, Tukey’s multiple comparisons shown). D Cell viability 16 h after palmitate exposure in C2C12 myoblasts expressing WT or K120Q p53 (Two-way ANOVA, treatment p < 0.0001 F(1,12) = 50.44, genotype p = 0.0361 F(1,12) = 5.56, significant Tukey’s multiple comparisons shown). E Cell viability in C2C12 myoblasts following palmitate exposure and co-incubation with vehicle (DMSO) or increasing concentrations of Z-DEVD-FMK (One-way ANOVA, p = 0.3282). F Schematic showing key regulatory points in the ferroptosis pathway. G Cell viability in C2C12 myoblasts following palmitate exposure and co-incubation with vehicle (DMSO) or increasing concentrations of Ferrostatin (One-way ANOVA, p = 0.0323 ×2 = 0.5337, significant Tukey’s multiple comparisons shown). H Cell viability in Control or HDAC4 and 5 overexpressing C2C12 myoblasts following exposure to increasing concentrations of Erastin (Two-way ANOVA, genotype p < 0.0001 F(1,18) = 293.7, treatment p < 0.0001 F(2,18) = 139.6, interaction p < 0.0001 F(2,18) = 50.88, significant Tukey’s multiple comparisons shown). I Glutamate (Glu), glutathione (GSH) and the GSH/Glu ratio in Control or HDAC4 and 5 overexpressing C2C12 myoblasts (Unpaired t-tests). J Glutathione peroxidase (GPX) activity in Control or HDAC4 and 5 overexpressing C2C12 myoblasts (Unpaired t-test). K H2O2 derived from mitochondria (mito), NADPH oxidases (NOX) and other sources in Control or HDAC4 and 5 overexpressing C2C12 myoblasts (Unpaired t-tests). Data are mean ± SEM, n = 4–10 biological replicates per group.