Table 1.
Relative binding affinity (RBA) and Ki of all compounds to ERα and ERβa.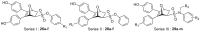
| Entry | Compd. | R1 | R2 | R3 |
Ki (nmol/L) |
RBA (%) |
α/β ratio | ||
|---|---|---|---|---|---|---|---|---|---|
| ERα | ERβ | ERα | ERβ | ||||||
| 1 | 26a | 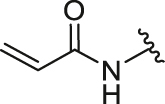 |
/ | / | 0.34 | 1.73 | 8.04 ± 0.87 | 3.86 ± 0.34 | 2.08 |
| 2 | 26b | 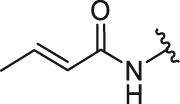 |
/ | / | 0.58 | 16.68 | 4.77 ± 0.16 | 0.40 ± 0.05 | 11.93 |
| 3 | 26c | 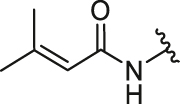 |
/ | / | 0.84 | 5.09 | 3.28 ± 0.17 | 1.31 ± 0.66 | 2.50 |
| 4 | 26d | 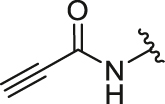 |
/ | / | 3.25 | 13.61 | 0.85 ± 0.08 | 0.49 ± 0.04 | 1.73 |
| 5 | 26e | 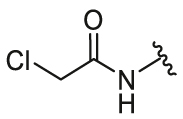 |
/ | / | 2.71 | 12.13 | 1.02 ± 0.13 | 0.55 ± 0.02 | 1.85 |
| 6 | 26f | 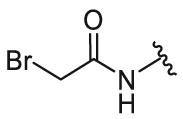 |
/ | / | 0.34 | 1.73 | 8.04 ± 0.37 | 3.86 ± 0.34 | 2.08 |
| 7b | 28a | 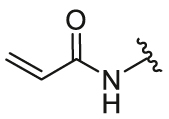 |
/ | / | 0.15 | 5.61 | 18.55 ± 0.34 | 1.19 ± 0.27 | 15.59 |
| 8b | 28b |  |
/ | / | 0.2 | 0.64 | 14.08 ± 0.25 | 10.40 ± 0.23 | 1.35 |
| 9b | 28c |  |
/ | / | 0.12 | 7.41 | 22.13 ± 0.92 | 0.90 ± 0.25 | 24.59 |
| 10b | 28d |  |
/ | / | 0.8 | 7.25 | 3.46 ± 0.02 | 0.92 ± 0.17 | 3.76 |
| 11b | 28e |  |
/ | / | 0.29 | 18.53 | 9.44 ± 0.40 | 0.36 ± 0.03 | 26.22 |
| 12b | 28f |  |
4-OH | / | 0.35 | 2.03 | 7.93 ± 0.34 | 3.29 ± 0.25 | 2.41 |
| 13 | 29a |  |
4-OH | CF3 | 0.14 | 0.99 | 20.03 ± 0.25 | 6.74 ± 0.21 | 2.97 |
| 14 | 29b |  |
4-OH | CF3 | 0.14 | 10.59 | 19.82 ± 0.19 | 0.63 ± 0.14 | 31.46 |
| 15 | 29c |  |
4-OH | CF3 | 0.09 | 10.93 | 30.44 ± 1.13 | 0.61 ± 0.11 | 49.90 |
| 16c | 29c′ |  |
4-OH | CF3 | 0.18 | 3.25 | 15.12 ± 0.31 | 2.05 ± 0.45 | 7.37 |
| 17 | 29d |  |
4-Me | CF3 | 0.43 | 4.87 | 6.49 ± 0.21 | 1.37 ± 0.11 | 4.74 |
| 18 | 29e |  |
H | CF3 | 0.29 | 23.82 | 9.50 ± 0.09 | 0.28 ± 0.05 | 33.9 |
| 19 | 29f |  |
4-OMe | CF3 | 0.19 | 4.07 | 14.48 ± 0.65 | 1.64 ± 0.45 | 8.83 |
| 20 | 29g |  |
4-F | CF3 | 0.37 | 6.54 | 7.52 ± 0.29 | 1.02 ± 0.13 | 7.37 |
| 21 | 29h |  |
3-OH | CF3 | 0.35 | 15.16 | 7.89 ± 0.11 | 0.44 ± 0.02 | 17.93 |
| 22 | 29i |  |
4-OH | CH3 | 0.09 | 1.3 | 29.85 ± 1.09 | 5.12 ± 0.39 | 5.83 |
| 23 | 29j |  |
4-OMe | CH3 | 0.63 | 13.08 | 4.36 ± 0.33 | 0.51 ± 0.09 | 8.55 |
| 24 | 29k | 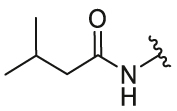 |
4-OH | CF3 | 1.15 | 4.6 | 2.41 ± 0.21 | 1.45 ± 0.12 | 1.66 |
| 25b | 29l | NH2 | 4-OH | CF3 | 1.14 | 2.84 | 2.43 ± 0.27 | 2.35 ± 0.33 | 1.03 |
| 26 | 29m | OH | 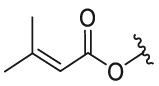 |
CF3 | 0.36 | 8.78 | 7.77 ± 0.43 | 0.76 ± 0.15 | 10.22 |
| 27 | 5 | 0.33 | 2.29 | 8.25 ± 0.67 | 2.91 ± 0.55 | 1.41 | |||
| 28d | 6a | 0.34 | 1.73 | 1.05 ± 0.16 | 0.10 ± 0.02 | 10.5 | |||
Relative binding affinity (RBA) values are the mean ± SD of at least three parallel tests. The RBA values: IC50estradiol/IC50compound × 100 ± the range (RBAestradiol = 100%). A high RBA means that the compound binds well to the ER and a low RBA means that the compound binds poorly to the ER. Ki = (100/RBA) × Kd. For estradiol, the Kd value was 2.76 and 6.67 nmol/L for ERα and ERβ, respectively.
Series II compounds were mixtures of regioisomers.
Compound 29c′ was regioisomer of 29c.
6a was a derivative of OBHSA, X = NCH2CF3, R = 4-OH.
