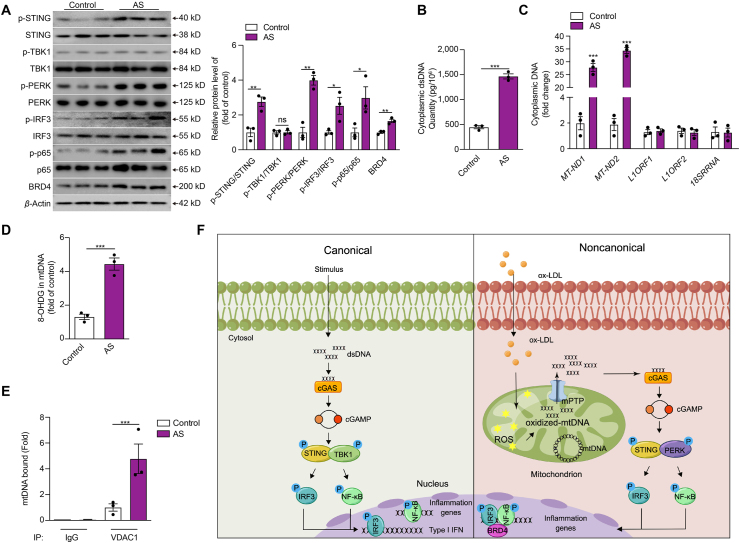
Figure 7.
The activation of the non-canonical STING-PERK pathway in human atherosclerotic plaques. (A–E) The protein level of the non-canonical STING–PERK pathway (A), the cytoplasmic dsDNA concentration (B), the cytoplasmic levels of mtDNA, nuclear LINE1 elements or RNA 18S (C), the mtDNA oxidation (D), mtDNA binding to VDAC1 (E) in the aortic tissues from patients with and without atherosclerosis. (F) The proposed model of non-canonical STING–PERK pathway activation and development of atherosclerosis. ox-LDL damages mitochondria of ECs and increases the generation of ROS, which oxidizes the mtDNA. The oxidized mtDNA interacts with VDAC1 and induces oligomerization of VDAC1 to form mPTP, releasing mtDNA to the cytosol. DNA binds and activates DNA sensor cGAS through conformational changes, producing cGAMP from ATP and GTP to activate the adaptor STING. STING dimerizes and recruits PERK to phosphorylate and activate transcription factors IRF3 and NF-κB. Meanwhile, STING regulates the formation of the transcriptional complex of super-enhancer on the proximal promoter regions of proinflammatory cytokines, including p-IRF3, p-p65, and BRD4 via the condensation characteristic of BRD4 to promote the transactivation of proinflammatory cytokines. Data are shown as mean ± SEM, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001; ns, no significance.