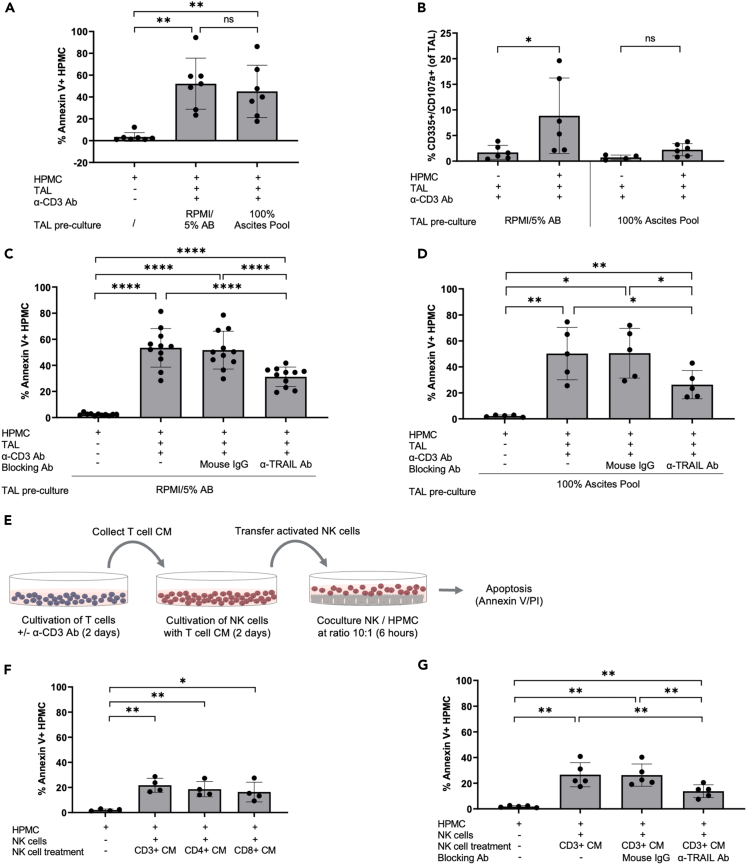
Figure 2.
Ascites blocks NK cell degranulation but not TRAIL-dependent HPMC apoptosis
(A and B) TAL were pre-cultured in RPMI/5% AB media or 100% ascites pool (+/− α-CD3 Ab stimulation) prior to co-culture with HPMC. (A) Apoptosis of HPMC is shown as percentage of annexin V+ cells after gating on CD45−cells (n = 7 patients). (B) Degranulating NK cells in response to HPMC were measured via flow cytometry and depicted as percentage of CD335+/CD107a+ cells (n = 6 patients).
(C and D) TAL were pretreated with α-TRAIL blocking Ab prior to HPMC co-culture. An irrelevant mouse IgG was included as control. Experiments were conducted with TAL cultured in RPMI/5% AB media (n = 11 patients) (C) and 100% ascites pool (n = 5 patients) (D). The amount of Annexin V+ HPMC was determined as described previously.
(E) Schematic representation of co-culture experiments applying T cell CM for NK cell activation. CM were collected from CD3+, CD3+/CD4+, and CD3+/CD8+ T cell subsets cultured in media +/− α-CD3 Ab stimulation. Purified NK cells were then stimulated with T cell CM prior to HPMC co-cultures.
(F) The amount of apoptotic HPMC induced by NK cells activated with CM of CD3+ T cells was compared with CM of CD3+/CD4+ and CD3+/CD8+ T cells (n = 4 matched pairs of different patients).
(G) Analysis of TRAIL signaling was performed by applying an α-TRAIL blocking Ab to NK cells stimulated with CD3+ T cell CM (α-CD3 Ab activated) prior to the co-culture with HPMC (n = 5 patients). An irrelevant mouse IgG was included as control.
The mean is shown by horizontal bars or boxes; vertical error bars represent the standard deviation in A–D, F, and G. ∗ FDR < 0.05; ∗∗ FDR < 0.01; ∗∗∗ FDR < 0.001; determined by paired t test and Benjamini-Hochberg adjustment (ns, not significant).