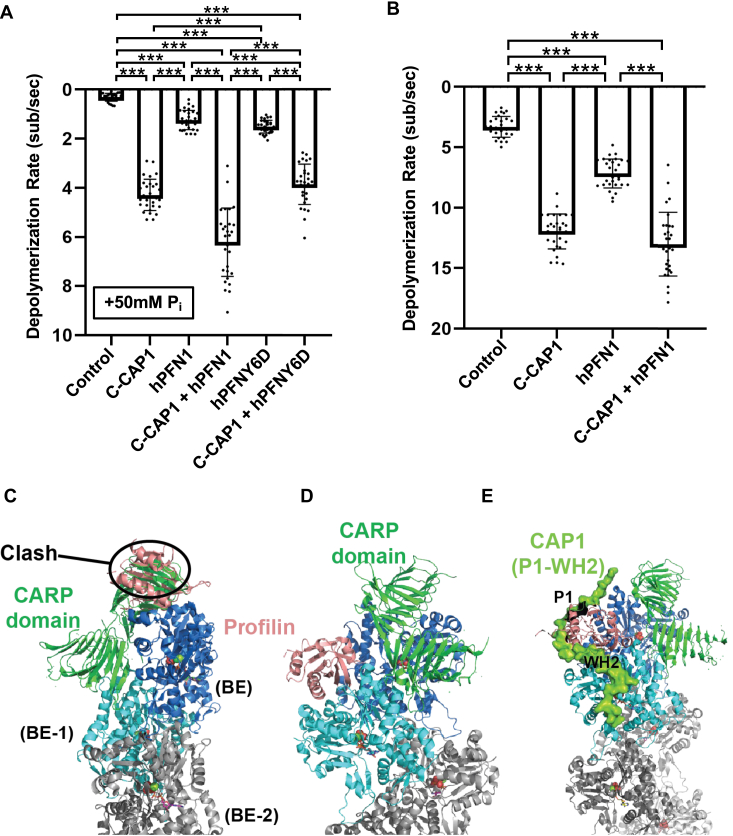
Figure 4.
CAP and profilin directly collaborate in promoting barbed end depolymerization.A, rates of barbed end depolymerization in the presence of 10 μM C-CAP1 and 10 μM WT PFN1 or mutant PFNY6D in mf-TIRF assays; all reactions include 50 mM inorganic phosphate (Pi). The data (mean ± SD) are from three independent replicates (n = 30 filaments total per condition) ∗∗∗, one-way ANOVA followed by Tukey’s multiple comparisons test to determine significance between indicated conditions (p < 0.0001). B, rates of barbed end depolymerization in the presence of 1 μM C-CAP1 and 10 μM human profilin (PFN1). The data (mean ± SD) are from three independent replicates (n = 30 filaments total per condition). ∗∗∗, one-way ANOVA followed by Tukey’s multiple comparisons test to determine significance between indicated conditions (p < 0.0001). C, to produce the working models shown in panels C–E, we docked the CARP domain as in the figure and used a profilin-actin cocrystal structure (PDB ID#: 2BTF) to position profilin on the actin filament model, aligning the actin from the cocrystal structure to the terminal actin (BE). The first model (panel C) shows that profilin and the CARP domain cannot both bind to the ultimate actin subunit (BE), as they have a steric overlap that includes most of the volume of profilin. D, in this model, profilin binds to the penultimate actin protomer (BE-1) without overlapping with the CARP domain bound to the ultimate subunit (BE). There is a minor overlap of profilin with the ‘hydrophobic plug’ of the terminal actin protomer, but this part of actin should be dynamic when not buried in the filament and could relax out of the minor apparent steric clash. E, using AlphaFold2, we generated a working model for how the CAP1 P1-WH2 region may interact with the barbed end, with the P1 motif bound to profilin and the WH2 domain bound to actin. When aligned to the penultimate actin subunit (BE-1), the P1-WH2 region has no steric clashes with profilin, actin, or the CARP domain. CAP, cyclase-associated protein; CARP, CAP and RP2 domain; C-CAP, C-terminal half of CAP; mf-TIRF, microfluidics-assisted total internal reflection fluorescence; WH2, Wiskott Aldrich syndrome 2.