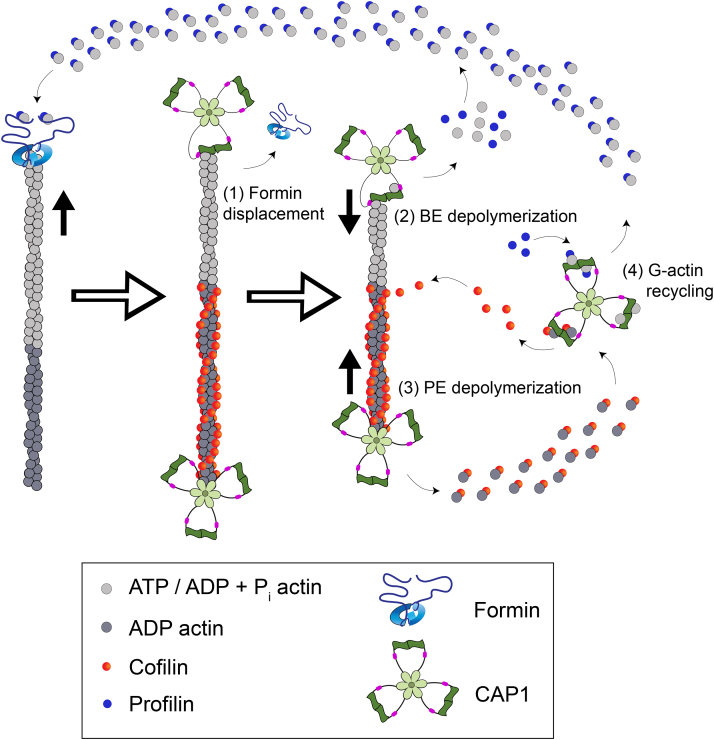
Figure 6.
Model summarizing new and established roles of CAP in promoting actin turnover. In this study, we have established that (1) CAP (green) promotes the displacement of formins (blue) from growing barbed ends and (2) that C-terminal domains of CAP (WH2 and CARP) interact with the barbed end (BE) to attenuate growth and promote depolymerization. Further, we have shown that CAP directly collaborates with profilin in promoting BE depolymerization through profilin interactions with the proline-rich P1 region of CAP. Previously, we and others established that (3) the N-terminal HFD domains of CAP interact with the pointed ends (PEs) of actin filaments where they synergize with cofilin (red circles) to promote PE depolymerization (54, 55), and (4) C-terminal WH2 and CARP domains of CAP recycle actin monomers, by binding to ADP-G-actin with high affinity, displacing cofilin, catalyzing nucleotide exchange (ATP for ADP) on G-actin, and handing off ATP-G-actin to profilin for new rounds of assembly (18, 49, 53, 57, 59, 60, 71). For simplicity, the ability of N-CAP to bind the sides of actin filaments (using its HFD domains) and enhance cofilin-dependent severing (18, 50, 79, 106) is not depicted in this cartoon but is likely to also contribute to F-actin disassembly. Because cellular concentrations of CAP are relatively high (6–7 μM; (43, 62)), we expect that there are sufficient CAP levels for most of these functions to be occurring simultaneously in cells. CAP, cyclase-associated protein; CARP, CAP and RP2 domain; HFD, helical folded domain; N-CAP, N-terminal half of CAP; WH2, Wiskott Aldrich syndrome 2.