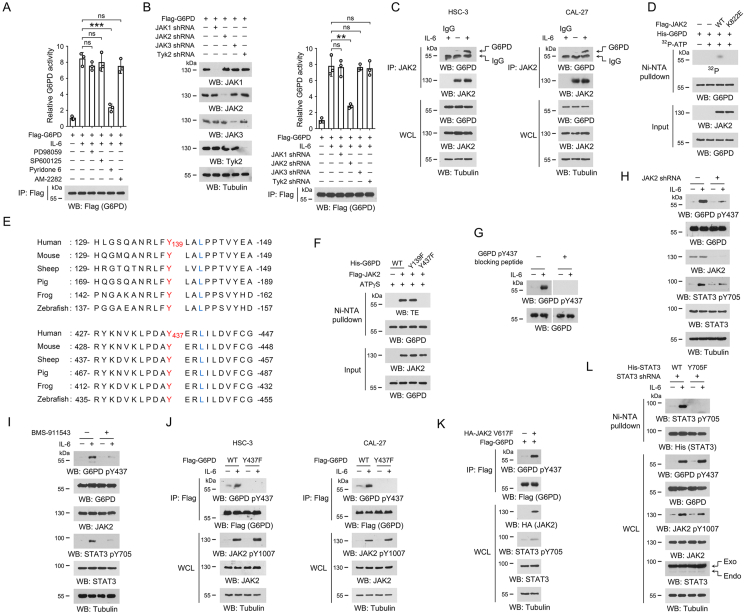
Figure 2.
JAK2 phosphorylates G6PD Y437 under IL-6 treatment.
(A-D, F-L) Immunoblotting analyses were performed using the indicated antibodies.
(A) HSC-3 cells were pre-treated with 20 μM PD98059, 20 μM SP600125, 2 μM Pyridone 6, or 1 μM AM-2282 for 2 h, respectively, and then treated with 50 ng/mL IL-6 for 1 h. Immunoprecipitations were performed with anti-Flag M2 antibody, and G6PD activity in the precipitates was examined. The data are presented as the mean ± SD from 3 independent experiments. ∗∗∗P < 0.001; ns, not significant.
(B) HSC-3 cells were transfected with the indicated shRNA and/or Flag-G6PD vector. 48 h after transfection, cells were treated with 50 ng/mL IL-6 for 1 h, and the cell lysates were used for immunoprecipitations with anti-Flag M2 antibody. The G6PD activity in the precipitates was examined. The data are presented as the mean ± SD from 3 independent experiments. ∗∗P < 0.01; ns, not significant.
(C) HSC-3 and CAL-27 cells were treated with or without 50 ng/mL IL-6 for 1 h, and co-immunoprecipitations were performed using the indicated antibodies. The data are obtained from 3 independent experiments. WCL, whole cell lysate.
(D) Purified His-G6PD protein was mixed with purified Flag-JAK2 or Flag-JAK2 K822E protein in the presence or absence of [γ-32P]-ATP for an in vitro kinase assay. Ni-NTA pulldown was performed, and the radioactivity in the precipitates was measured by autoradiography. The data are obtained from 3 independent experiments.
(E) Alignment analyses of G6PD Y139 or Y437 were performed using the G6PD protein sequences from the indicated species. Y139 and Y437 were shown in red, and the residues at +3 site that are required for JAK2 substrate consensus were labeled in blue.
(F) Purified WT His-G6PD protein or the indicated mutant proteins were incubated with purified Flag-JAK2 protein in the presence of ATPγS for an in vitro kinase assay. Ni-NTA pulldown was performed, and phosphorylation was detected using the thiophosphate ester (TE)-specific antibody. The data are obtained from 3 independent experiments.
(G) HSC-3 cells were treated with 50 ng/mL IL-6 for 1 h. G6PD Y437 phosphorylation was examined by immunoblot in the presence or absence of G6PD pY437 blocking peptide. The data are obtained from 3 independent experiments.
(H) HSC-3 cells with expression of JAK2 shRNA were treated with 50 ng/mL IL-6 for 1 h. The data are obtained from 3 independent experiments.
(I) HSC-3 cells were pre-treated with 2 μM BMS-911543 for 2 h, and then treated with 50 ng/mL IL-6 for 1 h. The data are obtained from 3 independent experiments.
(J) HSC-3 and CAL-27 cells with expression of WT Flag-G6PD or Flag-G6PD Y437F were incubated with 50 ng/mL IL-6 for 1 h, and immunoprecipitation was then performed with anti-Flag M2 antibody. The data are obtained from 3 independent experiments.
(K) HSC-3 cells with expression of Flag-G6PD and/or HA-JAK2 V617F, and immunoprecipitation was then performed with anti-Flag M2 antibody. The data are obtained from 3 independent experiments.
(L) We stably expressed STAT3 shRNA in HSC-3 cells, and then stably expressed WT His-STAT3 or His-STAT3 Y705F. Cells were incubated with 50 ng/mL IL-6 for 1 h, and Ni-NTA pulldown was performed. STAT3 shRNA targets the non-coding region. The data are obtained from 3 independent experiments.