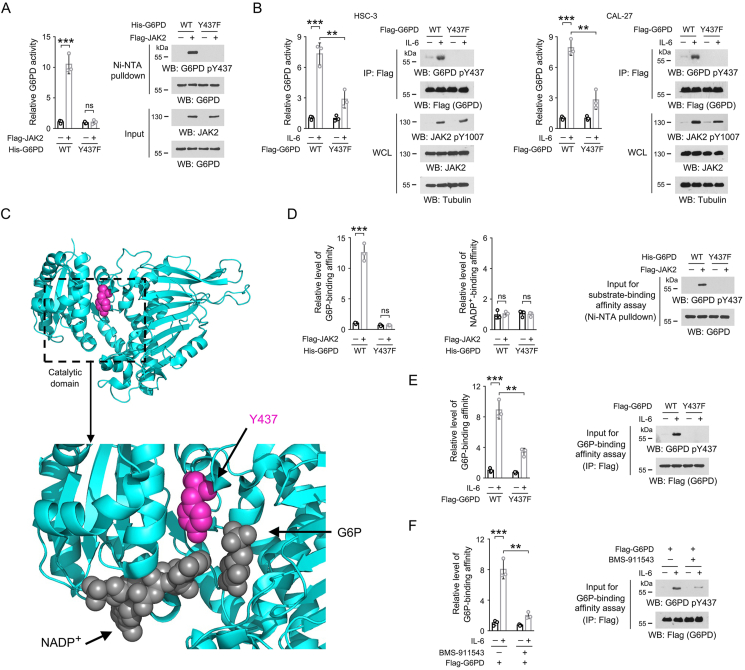
Figure 3.
JAK2-dependent Y437 phosphorylation activates G6PD by facilitating G6PD binding with G6P.
(A, B, D-F) Immunoblotting analyses were performed using the indicated antibodies.
(A) In vitro kinase assay was performed by mixing bacterially purified WT His-G6PD or Y437F mutant protein with purified Flag-JAK2 protein. Ni-NTA pulldown was performed, and the G6PD enzymatic activity in the precipitates was examined. The data are presented as the mean ± SD from 3 independent experiments. ∗∗∗P < 0.001; ns, not significant.
(B) HSC-3 and CAL-27 cells expressing WT Flag-G6PD or Flag-G6PD Y437F were treated with 50 ng/mL IL-6 for 1 h. Flag-G6PD proteins were precipitated from cell lysates, and the G6PD enzymatic activity in the precipitates was examined. The data are presented as the mean ± SD from 3 independent experiments. WCL, whole cell lysate; ∗∗P < 0.01, ∗∗∗P < 0.001.
(C) The catalytic domain of human G6PD protein (PDB code: 7UAL) was boxed and enlarged, and the binding sites for NADP+ and G6P are shown. The side chain of Y437 was shown in lightmagenta, and NADP+ and G6P were shown in dark grey.
(D) Bacterially purified WT His-G6PD or Y437F mutant protein was incubated with purified Flag-JAK2 for an in vitro kinase assay. Ni-NTA pulldown was performed, and the binding between G6PD protein and NADP+ or G6P was measured. The data are presented as the mean ± SD from 3 independent experiments. ∗∗∗P < 0.001; ns, not significant.
(E) HSC-3 cells expressing WT Flag-G6PD or Flag-G6PD Y437F were treated with 50 ng/mL IL-6 for 1 h. Flag-G6PD proteins were precipitated from cell lysates, and the binding between G6PD protein and G6P was examined. The data are presented as the mean ± SD from 3 independent experiments. ∗∗P < 0.01, ∗∗∗P < 0.001.
(F) HSC-3 cells with expression of WT Flag-G6PD were pre-treated with 2 μM BMS-911543 for 2 h, and then treated with 50 ng/mL IL-6 for 1 h. Flag-G6PD proteins were precipitated from cell lysates, and the binding between G6PD protein and G6P was assessed. The data are presented as the mean ± SD from 3 independent experiments. ∗∗P < 0.01, ∗∗∗P < 0.001.