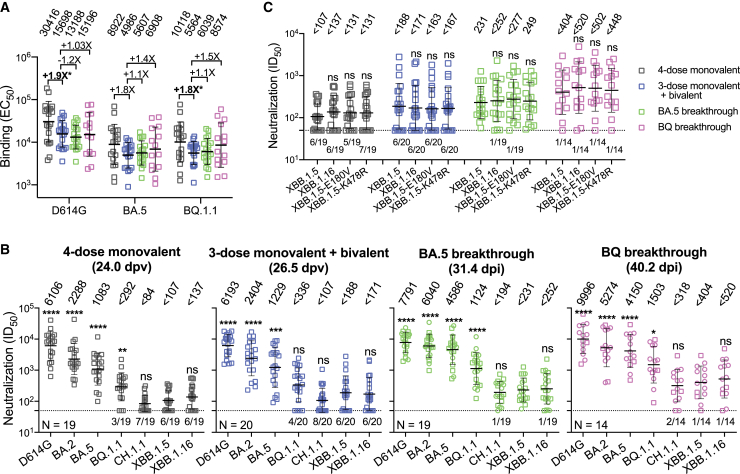
Figure 1.
SARS-CoV-2 antibody responses to D614G and Omicron subvariants following monovalent booster, bivalent booster, or breakthrough infection
(A) EC50 titers of binding antibodies in the serum samples from participants who received four doses of a monovalent mRNA vaccine (4-dose monovalent), three doses of a monovalent mRNA vaccine followed by one dose of a bivalent vaccine (3-dose monovalent + bivalent), and experienced BA.5 (BA.5 breakthrough) or BQ (BQ breakthrough) breakthrough infections after two to four doses of vaccine.
(B) ID50 titers of neutralizing antibodies in the serum samples from “4-dose monovalent,” “3-dose monovalent + bivalent,” “BA.5 breakthrough,” and “BQ breakthrough” cohorts against D614G and Omicron subvariants. Breakthrough serum samples were separated into two sub-groups using two distinct symbols. Square symbols indicate samples from individuals who received three doses of monovalent vaccines followed by a breakthrough infection. Round symbols indicate samples from individuals who received four or five doses of monovalent vaccines and subsequently had a breakthrough infection. dpv, days post last vaccination; dpi, days post infection. The numbers in parentheses represent the mean days post last vaccination (dpv) or the mean days post infection (dpi).
(C) Serum-neutralizing ID50 titers against XBB.1.5, XBB.1.16, and XBB.1.5 carrying the individual spike mutations found in XBB.1.16. Values above symbols indicate the geometric mean EC50 or IC50 titers for each cohort. The neutralization assay limit of detection (LOD) is 50 (dotted line), and the number of samples below the LOD is denoted above the x axis. Comparisons were made against “3-dose monovalent + bivalent” cohort (A) or XBB.1.5 (B and C) by Mann-Whitney tests. ns, not significant; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001. Sample sizes (n) are shown in (B). See also Tables S1 and S2.