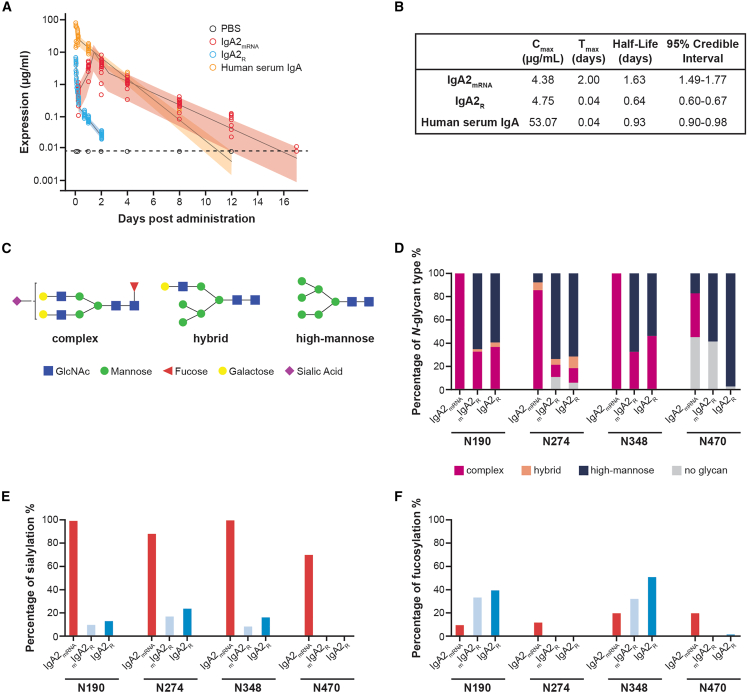
Figure 2.
Characterization of IgA2mRNA serum half-life and site-specific glycosylation patterns in vivo as compared to IgA2R
BALB/c mice were injected intravenously with 1 mg/kg formulated IgA2mRNA, 5 mg/kg Sal4 IgA2R, or 4.5 mg/kg IgA isolated from human serum. Concentrations of antibody were measured in serum over time by isotype-specific ELISA and modeled using a flexible linear mixed-effects model.
(A and B) Observed animal-level expression by ELISA and (A) model-based estimates of the mean and 95% credible interval and (B) model-based half-life estimates with 95% credible intervals are shown.
(C) Examples of three main types of N-glycan structure: complex, hybrid, and high mannose.
(D) IgA2 N-glycan compositions observed at 4 asparagine residues (N190, N274, N348, and N470).
(E) Sialylation of IgA2 asparagine residues, expressed as a percentage of complex and hybrid glycans that are sialylated.
(F) Fucosylation of IgA2 asparagine residues, expressed as a percentage of complex, hybrid, and high-mannose glycans that are fucosylated. Consistent detection of glycopeptides was observed across three technical executions in a serum pool from 50 animals.