Abstract
In some patients with acute respiratory distress syndrome (ARDS), a paradoxical improvement in respiratory system compliance (CRS) has been observed when assuming a supine (head of bed [HOB] 0°) compared with semirecumbent (HOB 35–40°) posture. We sought to test the hypothesis that mechanically ventilated patients with ARDS would have improved CRS, due to changes in ventilation distribution, when moving from the semirecumbent to supine position. We conducted a prospective, observational ICU study including 14 mechanically ventilated patients with ARDS. For each patient, ventilation distribution (assessed by electrical impedance tomography) and pulmonary mechanics were compared in supine versus semirecumbent postures. Compared with semirecumbent, in the supine posture CRS increased (33 ± 21 vs. 26 ± 14 mL/cm H2O, p = 0.005), driving pressure was reduced (14 ± 6 vs. 17 ± 7 cm H2O, p < 0.001), and dorsal fraction of ventilation was decreased (48.5 ± 14.1% vs. 54.5 ± 12.0%, p = 0.003). Posture change from semirecumbent to supine resulted in a favorable physiologic response in terms of improved CRS and reduced driving pressure—with a corresponding increase in ventral ventilation, possibly related to reduced ventral overdistension.
Keywords: acute respiratory distress syndrome, electrical impedance tomography, mechanical ventilation, respiratory system compliance
KEY POINTS
Question: In mechanically ventilated patients with acute respiratory distress syndrome (ARDS), how does posture, semirecumbent (head of bed 35–40°) versus supine (0°), affect respiratory system compliance (CRS) and distribution of ventilation?
Findings: CRS improved and driving pressure decreased in supine compared with semirecumbent posture. Ventilation increased ventrally when supine compared with semirecumbent posture.
Meanings: This study provides mechanistic data and insight into the observed paradoxical improvement in CRS with posture change from semirecumbent to supine in mechanically ventilated patients with ARDS. Supine position may result in improved distribution of ventilation compared with semirecumbent posture. Our findings suggest the need for additional research into optimal patient posture in this setting.
Optimal management in acute respiratory distress syndrome (ARDS) involves minimizing ventilator-induced lung injury (VILI) (1). Prone positioning is one intervention that takes advantage of lung mechanics and posture change to improve patient outcomes (2, 3). However, when not prone, semirecumbent position is still recommended in ARDS ostensibly to reduce the risk of aspiration. However, the semirecumbent posture may not optimize distribution of ventilation or respiratory system compliance (CRS). In some patients with ARDS, a paradoxical improvement in CRS has been observed when changing from semirecumbent (head of bed [HOB] 35–40°) to supine (HOB 0°) (4, 5). This observation suggests that body positioning could be used strategically in ARDS management.
Pleural pressure gradients and regional ventilation may provide insight into observed improvements in CRS when changing from semirecumbent to supine. Posture change alters pleural pressure gradients, modifying regional transpulmonary pressure (airway opening pressure minus pleural pressure) determining the ventilation distribution, and altering the alveolar overdistension/collapse balance.
We compared the effect of changing body posture (from semirecumbent to supine) on respiratory system mechanics and ventilation distribution using electrical impedance tomography (EIT) in patients with ARDS. We hypothesized that, compared with semirecumbent, the supine posture would increase CRS and reduce ventral overdistension.
MATERIALS AND METHODS
This single-center study was approved by the University of California, San Diego institutional review board (IRB) (study title: Ventilation and Perfusion in the Respiratory System, IRB number 210285, 80410, approval date July 16, 2021). Procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975. Patients older than 18 years admitted between August 2021 and August 2022, requiring invasive mechanical ventilation, and diagnosed with ARDS by Berlin criteria were included in the study. Exclusion criteria included: hemodynamic instability, conditions with confirmed or suspected increased intracranial pressure, and conditions precluding EIT use (e.g., pregnancy, pacemaker).
Ventilator settings and sedative administration, identical for both postures, were determined by treating clinicians. Semirecumbent and supine postures were HOB 35–40° and 0°, respectively. Initial position was randomized. Patients were maintained in each posture for 30 minutes after which plateau and driving pressures were obtained. Plateau pressure measurements were obtained in assist/control volume-cycled or volume-targeted modes of ventilation via inspiratory hold maneuver during nonspontaneous breathing. Two patients were on pressure-control ventilation and did not yield plateau pressure values. Available arterial blood gas values were recorded.
Each patient was fitted with an EIT belt placed between the fourth and fifth intercostal spaces. EIT data (50 Hz sampling rate) (Enlight 2100, Timpel, Brazil) were recorded to assess regional distribution of tidal ventilation in 32 × 32 pixel cross-sectional images. The outcome variable of interest was dorsal “fraction” of ventilation, defined as the sum of tidal impedance variation in the dorsal region divided by the global sum of tidal impedance variation for all pixels in the image (6, 7). Center of ventilation was defined as the coordinate along the ventral-dorsal axis with equal ventral and dorsal ventilation (8). Both metrics reflect the ventral-dorsal tidal ventilation distribution, with values greater than 50% indicating increased dorsal compared with ventral ventilation.
Data were analyzed via paired Student t test for statistical significance between postures (α = 0.05). Means and sds are reported. Normality of data was assessed using a Kolmogorov-Smirnov test.
RESULTS
A total of 14 patients were enrolled (five female patients, 62 ± 12 yr old; body mass index 29.5 ± 7.2 kg/m2; Pao2/Fio2 149 ± 49 mm Hg). EIT data were obtained in 13 of 14 patients, with 1 of 14 having image quality/interference issues. At baseline, tidal volume (Vt) was 6.5 ± 2. 1 mL/kg predicted body weight, and positive end-expiratory pressure (PEEP) was 7.9 ± 3.7 cm H2O. Twelve of 14 patients were ventilated using a volume-targeted mode and 2 of 14 were in pressure-control mode.
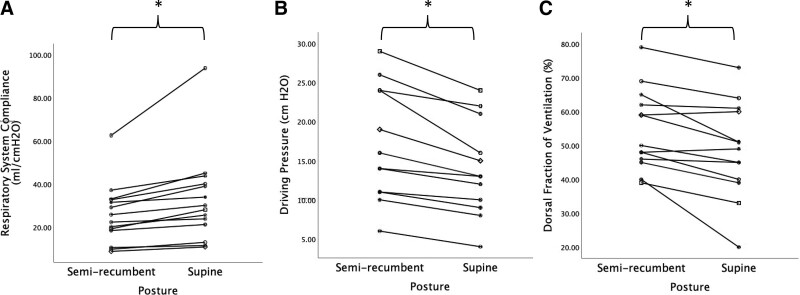
Supine CRS was increased compared with semirecumbent (33 ± 21 vs. 26 ± 14 mL/cm H2O, p = 0.005) (Table 1; Fig. 1). Driving pressure was reduced in the supine compared with semirecumbent position (14 ± 6 vs.17 ± 7 cm H2O, p < 0.001) (Table 1; Fig. 1). The dorsal fraction of ventilation was decreased in the supine compared with semirecumbent position (48.5 ± 14.1% vs. 54.5 ± 12.0%, p = 0.003), that is, ventral ventilation increased supine compared with semirecumbent (Table 1; Fig. 1). Center of ventilation was shifted ventrally in the supine position in comparison to the semirecumbent position (47.9 ± 3.8 vs. 49.5 ± 3.1, p = 0.005). Transition from semirecumbent to supine resulted in a favorable physiologic response, improved compliance, and/or decreased driving pressure, in 100% of patients (14/14).
TABLE 1.
Effect of Posture on Respiratory Mechanics, Distribution of Ventilation, and Hemodynamics
| Variables | Semirecumbent (35–40°) | Supine (0°) | p |
|---|---|---|---|
| Respiratory parameters | |||
| Respiratory system compliance, mL/cm H2O (n = 14) | 26 ± 14 | 33 ± 21 | 0.005 |
| Driving pressure (plateau pressure-positive end-expiratory pressure), cm H2O (n = 12a) | 17 ± 7 | 14 ± 6 | <0.001 |
| Electrical impedance tomography parameters (n = 13) | |||
| Dorsal fraction of ventilation, % | 54.5 ± 12.0 | 48.5 ± 14.1 | 0.003 |
| Center of ventilation, % | 49.5 ± 3.1 | 47.9 ± 3.8 | 0.005 |
| Hemodynamics (n = 14) | |||
| Spo2, % | 96.1 ± 3.2 | 95.8 ± 3.1 | 0.431 |
| Heart rate, beats/min | 95.4 ± 22.2 | 95.6 ± 23.7 | 0.916 |
| Mean arterial pressure, mm Hg | 80.7 ± 14.2 | 82.8 ± 14.6 | 0.475 |
| Respiratory rate, breaths/min | 25.4 ± 5.1 | 25.3 ± 4.7 | 0.752 |
| Arterial blood gas values/gas exchange (n = 4) | |||
| pH | 7.31 ± 0.07 | 7.39 ± 0.05 | 0.215 |
| Paco2, mm Hg | 54.8 ± 23.0 | 48.0 ± 11.4 | 0.374 |
| Pao2, mm Hg | 74.3 ± 12.8 | 98.5 ± 45.2 | 0.380 |
| Ratio of Pao2 to Fio2 | 131 ± 44 | 166 ± 35 | 0.161 |
Data presented as average ± sd unless noted as n (%).
Plateau pressure values used to calculate driving pressure were only recorded in patients in assist/control volume-cycled or volume-targeted modes of ventilation and were not recorded for two patients on pressure-control ventilation.
Figure 1.
Impact of posture on respiratory system compliance, driving pressure, and dorsal fraction of ventilation. A, Respiratory system compliance. B, Driving pressure. C, Dorsal fraction of ventilation. Individual patient data are identified by unique symbols and connected with solid lines. *p < 0.05 semirecumbent (35–40°) versus supine (0°).
DISCUSSION
In mechanically ventilated patients with ARDS changing posture from semirecumbent to supine resulted in significantly improved CRS and significant reduction in driving pressure, as previously reported (5). Compared with semirecumbent, the supine posture shifted regional ventilation ventrally, offering insight into why CRS improves with postural change toward supine. EIT is a noninvasive bedside approach to identify regions of the lung receiving tidal ventilation, based on relative impedance changes with each breath. If lung is overdistended or atelectatic throughout the respiratory cycle, EIT may not detect ventilation locally. Based on the baby lung ARDS concept (9), the ventral/nondependent lung is open while the dorsal/dependent lung is atelectatic. An increase in ventral ventilation when transitioning to the supine posture may suggest potential relief from initial ventral overdistention when semirecumbent (7). Although changes in regional atelectasis cannot be ruled out as a potential mechanism for our findings, the known effects of the abdomen on diaphragm and lung (10), short timeframe of our measurements, and rapid improvement in CRS suggest that it is less likely.
In the semirecumbent position, there is a pleural pressure gradient from apex to base, that is mainly gravitational (11, 12). This gradient leads to higher apical and lower basilar transpulmonary pressures, resulting in increased apical distension (possibly overdistension) compared with the base (12, 13). Furthermore, in the supine posture, the apex-to-base pleural pressure gradient is reduced with more uniformly distributed transpulmonary pressure throughout the lung (12, 13). We suggest that improvements in CRS when supine may ameliorate ventral overdistension when semirecumbent. If a reduction in overdistension were to occur (i.e., fewer alveoli on the flat part of the pressure–volume curve), for any given tidal volume, more air may be distributed to regions of the lung that can participate in gas exchange without leading to lung injury. The rapid response to supination further suggests pulmonary mechanics are sensitive to postural change, providing a simple means of clinical assessment for personalized ventilation.
It is important to highlight the complexities of pulmonary mechanics in ARDS as there may be alternative hypotheses for our findings. Although studies of body posture have been informative, other innovative ICU studies have also informed this discussion (14, 15). For example, Kummer et al (4) showed abdominal compression ostensibly led to decreased overdistension as evidenced by changes in the stress index. Like posture change, chest wall, and abdominal compression also result in changes to local pleural pressure. However, these findings were influenced by baseline PEEP levels, highlighting the complex variables influencing lung and chest wall interactions. Clearly, further study of body position would be of interest accounting for influences of ventilator settings, body habitus, local pleural pressures, fat distribution, etc.
Our study, in which posture changes affected a driving pressure reduction, may have broader implications since driving pressures less than 15 cm H2O are associated with decreased ARDS mortality (16). Traditionally, it is thought that semirecumbent position reduces the risk of ventilator-associated pneumonia (VAP), but this position does not appear to confer any mortality benefit (17). Although a reproducible reduction in driving pressure when supine may indicate a lung protective advantage and could challenge current standard of care, further studies are needed to determine if driving pressure thresholds are applicable across all postures. A reduction in driving pressure may not imply lung protection but rather reflect increased lung compliance as may be seen with tidal recruitment. It is important to keep in mind that a driving pressure cutoff of less than 15 cm H2O likely reflects measurements done in patients in the semirecumbent posture. It is unclear if the same driving pressure threshold would apply in the supine (0°) position. We suggest that future studies regarding ARDS mechanical ventilation strategies consistently report head of the bed angles at a minimum.
There are several limitations in our study including small sample size, variability in ventilator settings, and inconsistent use of neuromuscular blockade. Due to sample size, we cannot comment on differences across different severities/etiologies of lung injury, different ventilator modes, or baseline levels of PEEP. The study duration and duration of intervention were short, precluding conclusions regarding sustained impact on VILI or occurence rate of VAP. Additionally, EIT reflects a cross-sectional slice (thickness ~5–10 cm), representing changes in ventral to dorsal distribution of ventilation (18, 19). As such it may miss changes occurring at the base or apex. Further, we do not have ventilator waveform recordings to review to evaluate for evidence of overdistention or changes in stress index (14, 20). Nevertheless, this study provides novel insight into the possible mechanism behind the paradoxical improvement in CRS when changing posture from semirecumbent to supine via EIT-assessed distribution of ventilation.
CONCLUSIONS
Changing posture from semirecumbent to supine reduced driving pressure, increased CRS, and increased ventral ventilation as assessed by EIT. These findings require further study as they may have implications regarding how best to optimize mechanical ventilation and body position management of ARDS.
ACKNOWLEDGMENTS
The authors thank Stephen H. Loring, MD, for his helpful feedback regarding this work.
Footnotes
Drs. Pearce, Malhotra, Elliott, McGuire, and Butler participated in conception and design. Dr. Pearce participated in data acquisition. Drs. Pearce, Elliott, McGuire, Prisk, Goligher, Malhotra, and Butler participated in data analysis and interpretation. Drs. Pearce, Malhotra, Elliott, McGuire, Prisk, Goligher, and Butler participated in drafting and revising the article. Drs. Pearce, Malhotra, Elliott, McGuire, Prisk, Goligher, and Butler participated in the final article approval.
Drs. Pearce and McGuire received funding from the National Institutes of Health- National Heart Lung and Blood Institute (NIH-NHLBI) T32. Dr. Malhotra has received funding from the National Institutes of Health (NIH). Dr. Malhotra also reports income related to medical education from Livanova, Jazz, Zoll, and Eli Lilly. ResMed gave a philanthropic donation to University of California San Diego (UCSD). Dr. Malhotra is a co-founder of Healcisio which is a startup related to predictive analytics in sepsis. The remaining authors have disclosed that they do not have any conflicts of interest related to this work.
Clinicaltrials.gov identifier: NCT05081895.
REFERENCES
- 1.Slutsky AS: Lung injury caused by mechanical ventilation. Chest 1999; 116:9S–15S [DOI] [PubMed] [Google Scholar]
- 2.Katira BH, Osada K, Engelberts D, et al. : Positive end-expiratory pressure, pleural pressure, and regional compliance during pronation: An experimental study. Am J Respir Crit Care Med 2021; 203:1266–1274 [DOI] [PubMed] [Google Scholar]
- 3.Guerin C, Reignier J, Richard JC, et al. ; PROSEVA Study Group: Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368:2159–2168 [DOI] [PubMed] [Google Scholar]
- 4.Kummer RL, Shapiro RS, Marini JJ, et al. : Paradoxically improved respiratory compliance with abdominal compression in COVID-19 ARDS. Chest 2021; 160:1739–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marrazzo F, Spina S, Forlini C, et al. : Effects of trunk inclination on respiratory mechanics in patients with COVID-19-associated acute respiratory distress syndrome: Let’s Always Report the Angle! Am J Respir Crit Care Med 2022; 205:582–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brochard L, Yoshida T, Amato M: Simple electrical impedance tomography measures for the assessment of ventilation distribution. Am J Respir Crit Care Med 2020; 201:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshida T, Piraino T, Lima CAS, et al. : Regional ventilation displayed by electrical impedance tomography as an incentive to decrease positive end-expiratory pressure. Am J Respir Crit Care Med 2019; 200:933–937 [DOI] [PubMed] [Google Scholar]
- 8.van Heerde M, Roubik K, Kopelent V, et al. : Spontaneous breathing during high-frequency oscillatory ventilation improves regional lung characteristics in experimental lung injury. Acta Anaesthesiol Scand 2010; 54:1248–1256 [DOI] [PubMed] [Google Scholar]
- 9.Gattinoni L, Pesenti A: The concept of “baby lung”. Intensive Care Med 2005; 31:776–784 [DOI] [PubMed] [Google Scholar]
- 10.Yoshida T, Engelberts D, Otulakowski G, et al. : Continuous negative abdominal pressure: Mechanism of action and comparison with prone position. J Appl Physiol (1985) 2018; 125:107–116 [DOI] [PubMed] [Google Scholar]
- 11.Agostoni E, D’Angelo E: Comparative features of the transpulmonary pressure. Respir Physiol 1970; 11:76–83 [DOI] [PubMed] [Google Scholar]
- 12.D’Angelo E, Bonanni MV, Michelini S, et al. : Topography of the pleural pressure in rabbits and dogs. Respir Physiol 1970; 8:204–229 [DOI] [PubMed] [Google Scholar]
- 13.Mead J, Gaensler EA: Esophageal and pleural pressures in man, upright and supine. J Appl Physiol 1959; 14:81–83 [DOI] [PubMed] [Google Scholar]
- 14.Stavi D, Goffi A, Al Shalabi M, et al. : The pressure paradox: Abdominal compression to detect lung hyperinflation in COVID-19 acute respiratory distress syndrome. Am J Respir Crit Care Med 2022; 205:245–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selickman J, Crooke PS, Tawfik P, et al. : Paradoxical positioning: Does “head up” always improve mechanics and lung protection? Crit Care Med 2022; 50:1599–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amato MB, Meade MO, Slutsky AS, et al. : Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015; 372:747–755 [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Li X, Yang Z, et al. : Semi-recumbent position versus supine position for the prevention of ventilator-associated pneumonia in adults requiring mechanical ventilation. Cochrane Database Syst Rev 2016:CD009946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabbani KS, Kabir AM, Kabir AM: Studies on the effect of the third dimension on a two-dimensional electrical impedance tomography system. Clin Phys Physiol Meas 1991; 12:393–402 [DOI] [PubMed] [Google Scholar]
- 19.Frerichs I, Amato MB, van Kaam AH, et al. ; TREND study group: Chest electrical impedance tomography examination, data analysis, terminology, clinical use and recommendations: Consensus statement of the TRanslational EIT developmeNt stuDy group. Thorax 2017; 72:83–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grasso S, Terragni P, Mascia L, et al. : Airway pressure-time curve profile (stress index) detects tidal recruitment/hyperinflation in experimental acute lung injury. Crit Care Med 2004; 32:1018–1027 [DOI] [PubMed] [Google Scholar]