Figure 3. Biochemical analyses of residue 417 in regulating the conformation of membrane-anchored full-length SARS-CoV-2 spike and of the functions of the spike in different conformations.
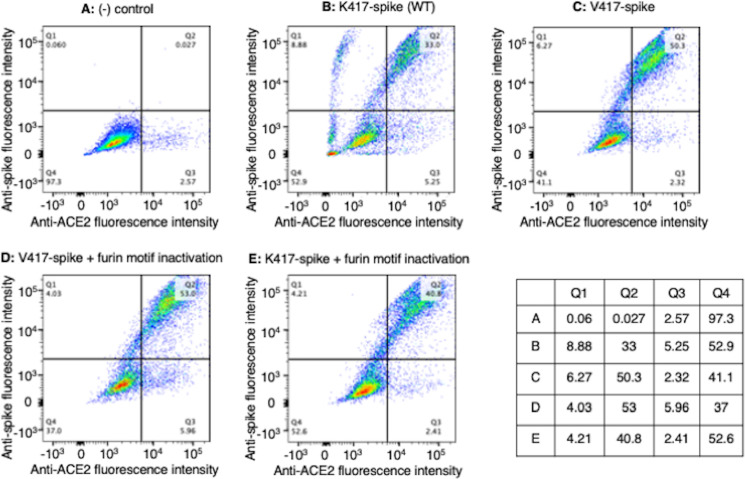
(A) Protein pull-down assay using recombinant human ACE2 as the bait and cell-surface-anchored full-length SARS-CoV-2 spike as the target. The spike contains either Lys417 (wild-type residue) or Val417 (mutant residue). Top: cell-surface-expressed SARS-CoV-2 spike. Middle: pull-down results using His6-tagged ACE2. Bottom: pull-down results using Fc-tagged ACE2 (Figure 3—source data 1). The expected molecular weights of SARS-CoV-2 spike monomer and S2 monomer are ~180 kDa and ~80 kDa, respectively. (B) Flow cytometry assay to detect the interactions between recombinant human ACE2 and cell-surface-anchored full-length SARS-CoV-2 spike (Figure 3—source data 2). The spike contains either Lys417 (wild-type residue) or Val417 (mutant residue) and contains either intact furin motif or inactivated furin motif. See Figure 3—figure supplement 1 for details of this experiment. Data are mean + SEM. A comparison (two-tailed Student’s t-test) was performed on data between indicated groups (n = 3). ***p<0.001. (C) SARS-CoV-2 pseudovirus entry into human-ACE2-expressing cells. The virus-surface-anchored spike contains either Lys417 (wild-type residue) or Val417 (mutant residue). Top: pseudovirus entry efficiency normalized against the expression level of the spike (see bottom) (Figure 3—source data 3). Bottom: SARS-CoV-2 spike in packaged pseudoviruses (Figure 3—source data 4). Data are mean + SEM. A comparison (two-tailed Student’s t-test) was performed on data between indicated groups (n = 8). ***p<0.001. All experiments in this figure were repeated independently three times with similar results.