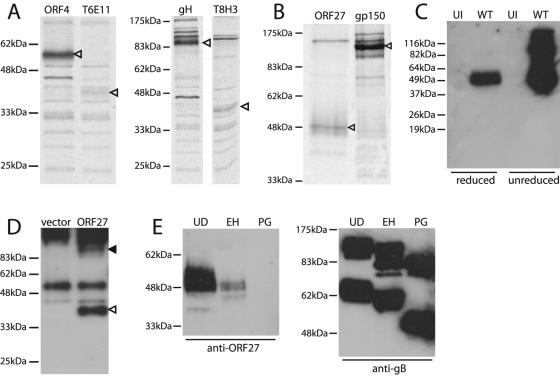
FIG. 2.
The mature ORF27 gene product is a 48-kDa glycoprotein. (A) BHK-21 cells were infected with MHV-68 (2 PFU/cell), and 18 h later, were labeled with [35S]cysteine-methionine for 1 h. ORF27 was then immunoprecipitated with the T6E11 or T8H3 MAb. Immunoprecipitations with the ORF4-specific MAb T3B8 and the gH-specific MAb 2G2 are shown for comparison. Arrowheads indicate specific precipitated bands. (B) For this experiment, the label was chased overnight with an excess of unlabeled cysteine-methionine. Virions were then recovered from cell-free supernatants by ultracentrifugation. ORF27 was immunoprecipitated from virion lysates with the T8A11 MAb. The immunoprecipitation of gp150 with MAb T4G2 is shown for comparison. Arrowheads indicate specific bands. (C) Lysates of purified MHV-68 virions (WT) or uninfected (UI) BHK-21 cells were immunoblotted with the 6D10 MAb. The samples were reduced or not with 5% 2-mercaptoethanol before electrophoresis, as indicated. (D) 293T cells were transfected with an ORF27 expression plasmid or an empty vector. Forty-eight hours later, the samples were immunoprecipitated with the T8A11 MAb and then immunoblotted with the T8H3 MAb, as the ORF27 protein was difficult to identify simply by immunoblotting transfected cell lysates. The open arrowhead indicates the major specific band. There was also a possible higher-molecular-mass, ORF27-specific band (filled arrowhead), but this was difficult to distinguish from the precipitating MAb. The samples were unreduced, so even though the band at 55 kDa corresponds to the IgG heavy chain, the bulk of the immunoglobulin ran intact at higher molecular masses. (E) Infected cell lysates were reduced, denatured, and then either left undigested (UD), digested with endoglycosidase H (EH), or digested with protein N-glycanase (PG). The samples were then immunoblotted with the T8H3 MAb (a similar pattern was observed with the 6D10 MAb). Immunoblotting of the same samples with the gB-specific MAb T7H9 provided a control of protein integrity.