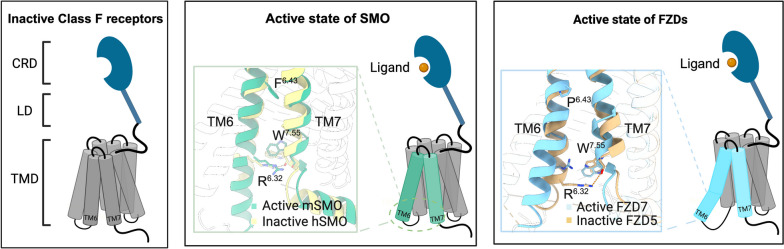
Fig. 6.
Conformational alterations during Class F receptor activation. Class F receptors are composed of a cystine-rich domain (CRD), linker domain (LD) and transmembrane domain (TMD), and the inactive state is shown on the left. When activated by the ligand, TM6 in SMO and FZDs undergoes an outward shift, and a hydrogen bond between the conserved residues R6.32 and W7.55 in the inactive receptor is disrupted. The difference between SMO and FZDs is that the TM6 in SMO exhibits a parallel outward shift, while FZDs achieve a similar displacement of its cytoplasmic segment through a helical kink. This difference may be caused by the conserved residue P6.43 in the FZDs (as opposed to F6.43 in SMO). PDB ID of these structures: active mSMO (6O3C), inactive hSMO (5I7D); active FZD7 (7EVW), inactive FZD5 (6WW2)