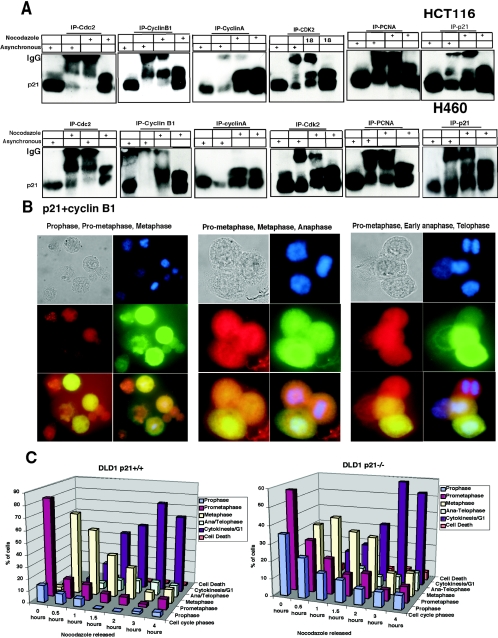
FIG.5.
Cyclin B1 interacts exclusively with hyperphosphorylated p21, whereas Cdc2 interacts preferentially with hyperphosphorylated p21 compared to hypo-phosphorylated p21. (A) Immunodepletion of asynchronous or G2/M-enriched H460 or HCT116 cells with various monoclonal antibodies (as indicated) and immunoblotting for p21 protein. Total cell extracts from asynchronous and nocodazole-treated cells are shown in each case to mark the migration of the hyper- and hypophosphorylated p21. Immunoprecipitation using anti-cyclin A, CDK2, or PCNA antibodies revealed similar association of these proteins with hyper- and hypo-phosphorylated p21. The p21 monoclonal antibody used for immunoprecipitation (rightmost panels) bound and precipitated both the hyper- and hypophosphorylated p21 proteins. (B) p21 colocalizes with cyclin B1 in the nucleus at G2/M. Eighteen-hour nocodazole-treated H460 cells released from microtubule inhibition for 0.5 h were fixed and simultaneously stained with human monoclonal p21 (red) and human cyclin B1 polyclonal antibodies (green). Prophase, prometaphase, and metaphase cells show milkish/pink granular structures over the chromatin material with nuclear p21 and cyclin B1 localization after cells were treated for 18 h with nocodazole or released from nocodazole for half an hour. In late anaphase or early telophase stage, only p21 was localized in the nucleoplasm (red), but cyclin B1 degraded. (C) Phosphorylated p21 acts as a positive regulator of the G2-prophase stage. Comparison of percentage of mitotic phases in nocodazole-treated or nocodazole-released cells at different time points using DLD1 p21−/− and p21+/+ cells is shown.