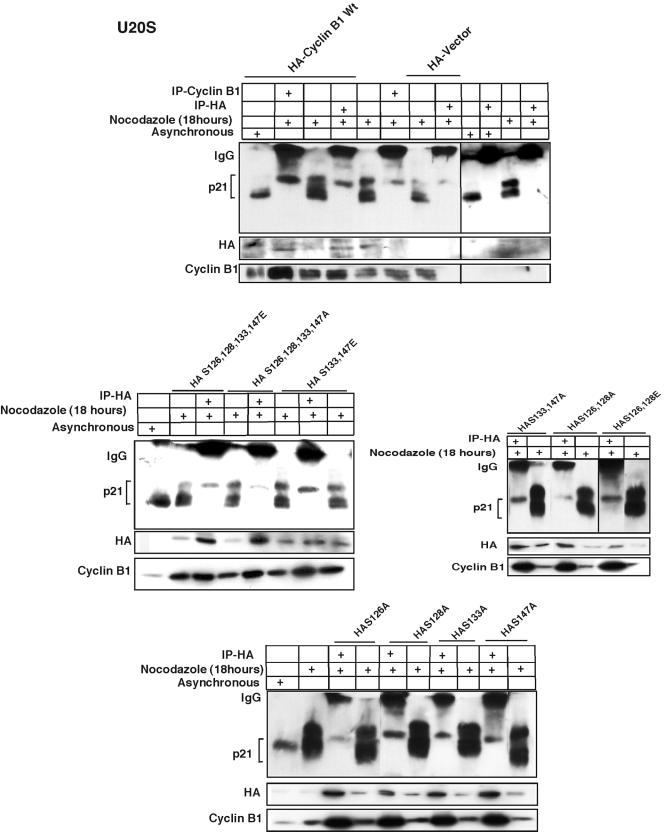
FIG. 7.
Phosphorylated p21 interacts specifically with serine 126 of cyclin B1. HA-tagged wild-type cyclin B1 constructs were transfected into U2OS cells for 24 h, and the cells were treated with nocodazole for another 18 h. Cells were then immunodepleted with HA monoclonal antibody and immunoblotted with p21 monoclonal antibody. Binding with hyperphosphorylated p21 protein was observed as with extracts immunodepleted with cyclin B1 antibody (Fig. 5A). Transfection of the HA-tagged cyclin B1 S126A S128A S133A S147A multiple serine-to-alanine substitution mutant very poorly bound with the phosphorylated p21, as did the S126A S128A mutant. There was no change in interaction with p21 with either the S126E S128E S133E S147E multiple serine-to-glutamic acid mutant or the S133A S147A, the S133E S147E, or the S126E S128E mutants. Phosphorylated p21 interacted very poorly with the cyclin B1 S126A mutant. However, the S128A, S133A, or S147A mutant construct interacted as normally as wild-type cyclin B1.