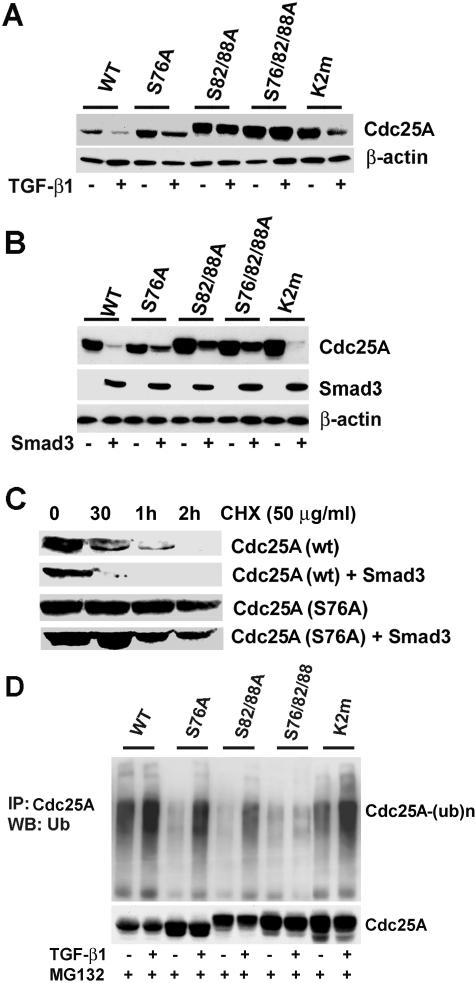
FIG. 4.
Phosphorylation around the DSG motif is involved in TGF-β-Smad3-mediated Cdc25A degradation. (A) Cdc25A mutants defective in phosphorylation around the DSG motif are refractory to TGF-β-mediated degradation. U2OS cells were transfected with wild type (WT) or the mutants of Cdc25A indicated in the panel and then treated with 10 ng of TGF-β1/ml between 9 and 24 h posttransfection. K2m, a K141A/E142A/N143A mutant defective in APC recognition. Cell lysates were analyzed by immunoblotting for the proteins indicated. (B) The DSG phosphorylation mutants of Cdc25A are refractory to Smad3-mediated degradation. U2OS cells were cotransfected with Smad3 and either wild type or the mutants of Cdc25A. Cell lysates at 24 h posttransfection were analyzed by immunoblotting. (C) Smad3 overexpression decreases stability of wild-type (wt) Cdc25A, whereas it affects the half-life of Cdc25A (S76A) only modestly. At 22 h posttransfection, cycloheximide (50 μg/ml) was added to the culture medium, and cells were further incubated for indicated times, followed by immunoblotting. (D) Phosphorylation around the DSG motif is critical for TGF-β1-mediated ubiquitination. U2OS cells were cotransfected with the indicated expression vectors and treated with or without TGF-β1 for 24 h in the presence of 1 μM MG132 between 9 and 24 h. Ubiquitinated Cdc25A [Cdc25A-(ub)n] was detected by immunoprecipitation (IP) followed by immunoblotting (WB).