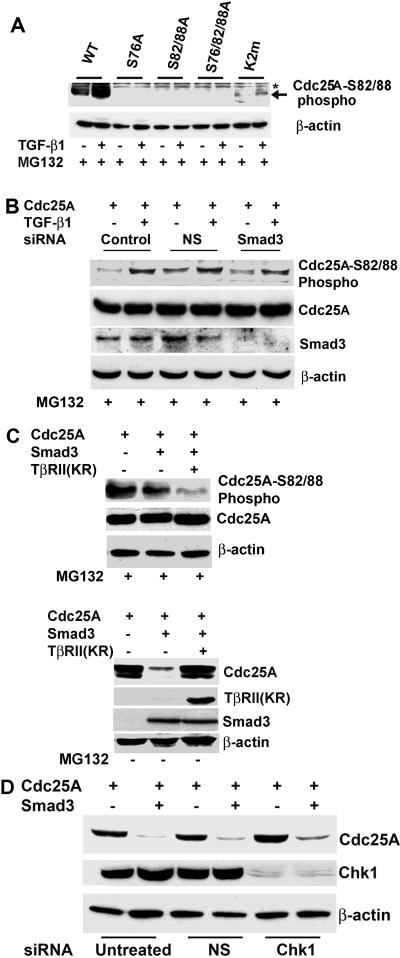
FIG. 5.
Cdc25A phosphorylation at the DSG motif is regulated by TGF-β signals independently of Smad3 levels. (A) TGF-β up-regulates phosphorylation at the DSG motif. U2OS cells were transfected with wild type (WT) and the mutants of Cdc25A and then treated with 10 ng of TGF-β1/ml for 24 h and with 1 μM MG132 between 9 and 24 h posttransfection, followed by immunoblotting with a polyclonal antibody specifically for Cdc25A phosphorylated at Ser82/Ser88. (B) Smad3 knockdown does not affect TGF-β-induced DSG phosphorylation. U2OS cells were transfected with Smad3 siRNA or NS dsRNA (NS), followed by transfection with Cdc25A and treatment with TGF-β and MG132 at 9 to 24 h posttransfection. Cells were then analyzed by immunoblotting with the indicated antibodies. (C) TGF-β receptor signaling is critical for DSG phosphorylation. (Upper panels) U2OS cells were transfected with indicated plasmids, followed by treatment with MG132 at 9 to 24 h posttransfection. TβRII(KR), a dominant-negative type II TGF-β receptor mutant. (Lower panels) The above experiment was performed in the absence of MG132. (D) Chk1 kinase is not critical for Smad3-induced Cdc25A degradation. U2OS cells were transfected with Chk1 siRNA or NS dsRNA, followed by transfection of Cdc25A in the presence or absence of Smad3. After 24 h, cells were subjected to immunoblotting for the indicated proteins.