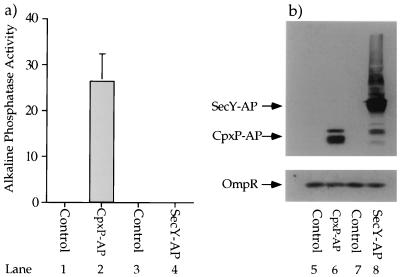
FIG. 5.
CpxP-AP possesses relatively high alkaline phosphatase activity. Strain SP627a (MC4100, ara74::cam) was transformed with pBR322 (control for pND24) (lanes 1 and 5), pND24 (produces CpxP-AP) (lanes 2 and 6), pBAD18 (control for pCH215) (lanes 3 and 7), and pCH215 (produces SecY-AP) (lanes 4 and 8). The transformants were grown in Luria broth containing 50 μg of ampicillin per ml supplemented with 0.4% l-arabinose to induce the synthesis of SecY-AP from pCH215. (a) The CpxP-AP fusion protein displays relatively high alkaline phosphatase activity. The alkaline phosphatase activities of each of these four transformant strains were determined in the presence of 5 mM IAA (lanes 1 to 4). (b) The amounts of alkaline phosphatase-cross-reacting species generated by the CpxP-AP and SecY-AP fusion proteins are comparable. Immunoblot analysis was performed on whole-cell protein extracts generated from SP627a transformed with pBR322, pND24, pBAD18, and pCH215 (lanes 5 to 8). The whole-cell extracts were separated by SDS-polyacrylamide gel electrophoresis (equal OD600 units were loaded in each lane) and subjected to immunoblot analysis with anti-alkaline phosphatase and anti-OmpR antisera. OmpR serves as an additional loading control.