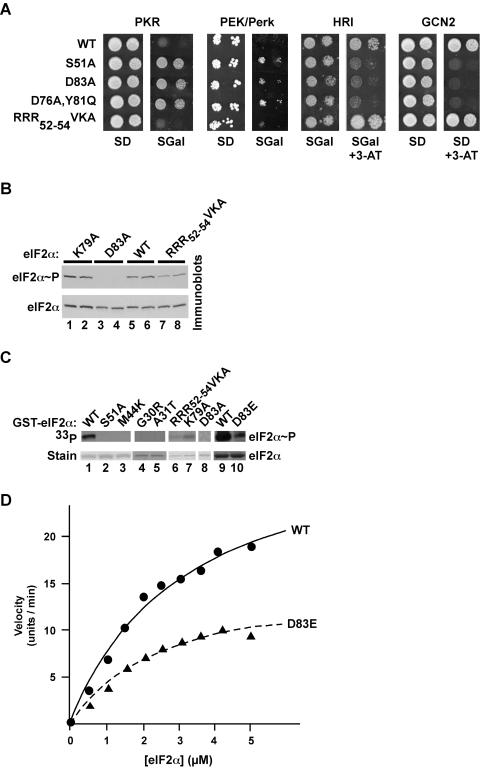
FIG. 6.
The kinases PKR, Perk/PEK, HRI, and GCN2 rely on the same eIF2α determinants for substrate recognition. (A) The eIF2α D83A mutation suppresses PKR, Perk/PEK, HRI, and GCN2 phenotypes in yeast. Plasmid p722, bearing GCN2, and plasmids p1420, p1246, and p551 (38), expressing human PKR, human HRI, and C. elegans PEK/Perk (residues 26 to 1077), respectively, under the control of a yeast GAL-CYC1 hybrid promoter, were introduced into derivatives of the gcn2Δ yeast strain H1925 expressing the indicated eIF2α alleles. Transformants were grown to saturation in minimal SD (2% glucose) medium, and 4 μl of serial dilutions (optical density at 600 nm, 1.0 and 0.1) was spotted onto minimal SD or SGal (10% galactose) medium or the same medium containing 10 mM 3-AT, as indicated. Plates were incubated at 30°C for 6 days. (B) Immunoblot analysis of eIF2α phosphorylation in yeast expressing PKR. The yeast strain H1925 expressing the indicated eIF2α alleles was transformed with the PKR expression vector p1420, and two independent transformants of each strain were grown to exponential phase in SDmedium and then shifted to SGR medium (containing 10% galactose plus 2% raffinose) for ∼18 h to induce PKR expression. WCEs were prepared, and total proteins were subjected to immunoblot analysis with antibodies against Ser51-phosphorylated eIF2α (upper panel) as described for Fig. 2A. The blot was stripped and reprobed with a polyclonal antiserum against total yeast eIF2α (lower panel). (C) In vitro kinase assay of PKR phosphorylation of eIF2α. Purified recombinant PKR was mixed with [γ-33P]ATP and the indicated recombinant GST-eIF2α fusion proteins purified from bacteria. Kinase reactions were resolved by SDS-PAGE, and gels were stained with Coomassie blue to detect GST-eIF2α (bottom panel), followed by autoradiography to visualize phosphorylated eIF2α (upper panel). (D) Kinetic analysis of PKR phosphorylation of eIF2α and eIF2α D83E. His6-eIF2α(1-200) and His6-eIF2α-D83E fusion proteins were purified from bacteria and examined for phosphorylation by purified PKR as described in Materials and Methods. Reaction products were resolved by SDS-PAGE, and the relative incorporation of phosphate into eIF2α was determined by using a PhosphorImager. The data are expressed in arbitrary units. Results are representative of at least three independent experiments.