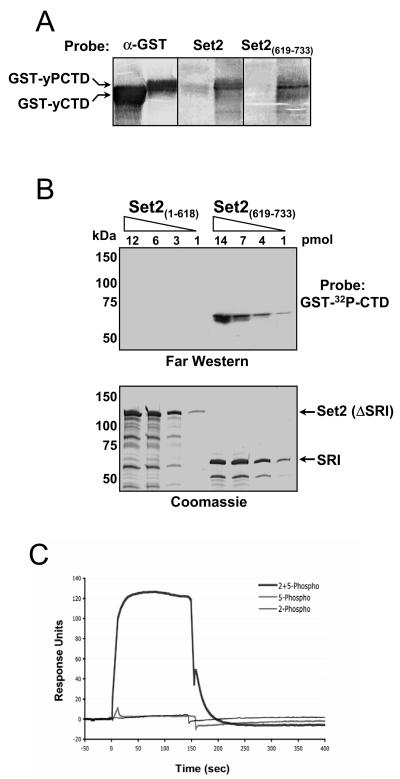
FIG. 3.
The SRI domain of Set2 binds synergistically to doubly modified CTD repeats. (A) Reverse far Western analysis. GST-yCTD and CTDK-I-phosphorylated GST-CTD (GST-yPCTD) fusion proteins were subjected to SDS-PAGE and transferred to nitrocellulose. Membranes were probed individually with purified recombinant full-length MBP-Set2 (Set2) or with MBP-SRI [Set2(619-733)], and the bound MBP fusions were detected with an anti-MBP antibody. As a control, a duplicate membrane was probed with an anti-GST antibody (α-GST) to demonstrate the presence of both GST-CTD fusion proteins. (B) Increasing amounts of two MBP fusion proteins [Set2(1-618) and Set2(619-733)] were resolved in two SDS-polyacrylamide gels; one gel was subjected to far Western analysis with GST-[32P]CTD as a probe, and the other was stained with Coomassie. (C) BIACORE analysis of the SRI domain. The MBP-SRI fusion protein [MBP-Set2(619-733)] was analyzed by surface plasmon resonance (BIACORE) for binding to distinct phosphorylated synthetic three-repeat CTD peptides. These peptides were either Ser5 phosphorylated (5-phospho), Ser2 phosphorylated (2-phospho), or both (2 + 5-phospho) in each repeat (see Materials and Methods). Response units, on the y axis, represent binding to the peptides. The binding response to a scrambled control peptide carrying six SerPO4residues (see Materials and Methods) has been subtracted from each of the three response curves. Only the peptide carrying both Ser2PO4 and Ser5PO4 in each repeat showed binding above control levels, and we estimate the affinity of this interaction (after subtraction of background binding to the control peptide) to be 6 μM.