Table 3.
Kinetic vs. thermodynamic study of various neocannabinoids with experimental selectivity ratios and DFT-calculated energies
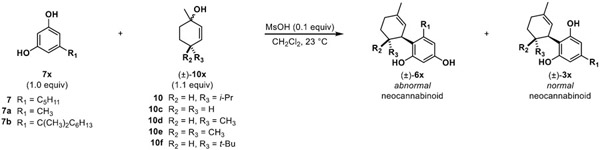
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Resorcinol 7x |
Allylic alcohol 10x |
(r.r.), 1 h (3x:6x)a |
(r.r.), 24 h (3x:6x)a |
Isolated product (±)-3x |
% Yield isolated (±)-3x, 24 h |
Computational results | |
| K3/k6 b | ΔG (kcal/mol)c | |||||||
| 1 | 7a | 10c | (1:3) | (1:1) | 3ac | 9% | 0.48 | 1.3 |
| 2 | 7a | 10d | (2:5) | (3:4) | 3ad | 37% | 0.24 | 1.7 |
| 3 | 7a | 10e | (1:10) | (3:10) | 3ae | 16% | 0.48 | 5.9 |
| 4 | 7a | 10 | (2:3) | (11:1) | 3a | 47% | 1.20 | 4.8 |
| 5 | 7a | 10f | (5:1) | (9:1) | 3af | 51% | 0.34 | 4.0 |
| 6 | 7 | 10c | (7:10) | (1:1) | 3c | 43% | 0.67 | 1.0 |
| 7 | 7 | 10d | (1:1) | (5:1) | 3d | 56% | 0.85 | 4.0 |
| 8 | 7 | 10e | (1:4) | (1:3) | 3e | 18% | 0.51 | 2.1 |
| 9 | 7 | 10 | (11:10) | (1:0) | 3 | 81% | 0.49 | 1.7 |
| 10 | 7 | 10f | (27:1) | (25:1) | 3f | 75% | 2.34 | 1.7 |
| 11 | 7b | 10c | (1:0) | (1:0) | 3bc | 98% | 4.52 | 7.3 |
| 12 | 7b | 10d | (1:0) | (1:0) | 3bd | 98% | 8.80 | 9.3 |
| 13 | 7b | 10e | (1:0) | (1:0) | 3be | 72% | 206.01 | 11.4 |
| 14 | 7b | 10 | (1:0) | (1:0) | 3b | 79% | 49.72 | 10.8 |
| 15 | 7b | 10f | (1:0) | (1:0) | 3bf | 56% | 117.65 | 11.9 |
Ratio determined by HPLC/ELSD/MS and 1H NMR analysis.
, where ΔΔG = G(6x‡) – G(3x‡), T = 298.15 K, and G(3/6x‡) is the Boltzmann-averaged free energy.
ΔG = G(6x) – G(3x), where G(3/6x) is the Boltzmann-averaged free energy.
