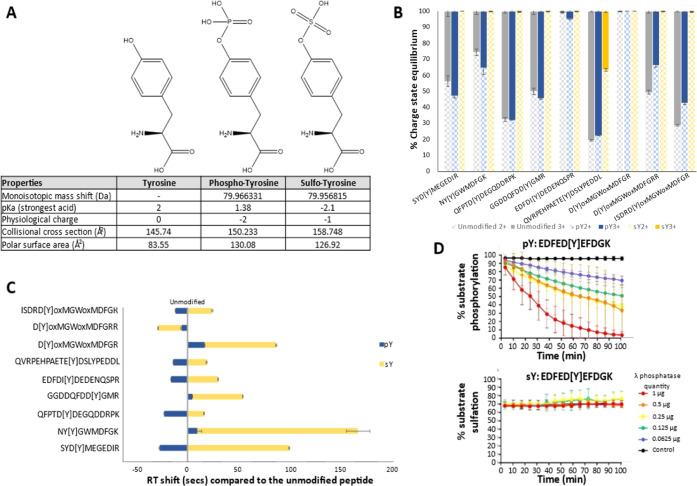
Figure 1.
Physiochemical properties of pY vs sY. (A) Chemical structure and properties of tyrosine, phospho-tyrosine, and sulfo-tyrosine. A table of the monoisotopic mass shift (Da) of the phosphate/sulfate moiety in comparison to tyrosine, the pKa value of the strongest acidic group, net charge influence at physiological pH (∼8), collisional cross section (Å2), and the polar surface area charge-distributed across (Å2) are presented. Data were compiled from the 2022 Human Metabolome Database. (B) Charge state distribution of our standard panel of peptides in an unmodified, phosphorylated, or sulfated state. Modification site denoted by [Y], ox = oxidized residue. Error bars represent SE from N = 10 replicates. (C) Scaled RT shifts of pY- and sY-peptides. Normalized RT shifts (s) are plotted with reference to the RT of the unmodified peptide counterpart. Modification site denoted by [Y], ox = oxidized residue. Error bars represent SE from N = 10 replicates. (D) Real-time λ phosphatase assay to study peptide dephosphorylation or desulfation. Identical fluorescent peptide sequences containing either pY or sY were incubated with stated quantities of λ phosphatase, and the level of modification reversal (dephosphorylation or desulfation) was determined over time (min) using a ratiometric real-time assay. Error bars represent S.D.