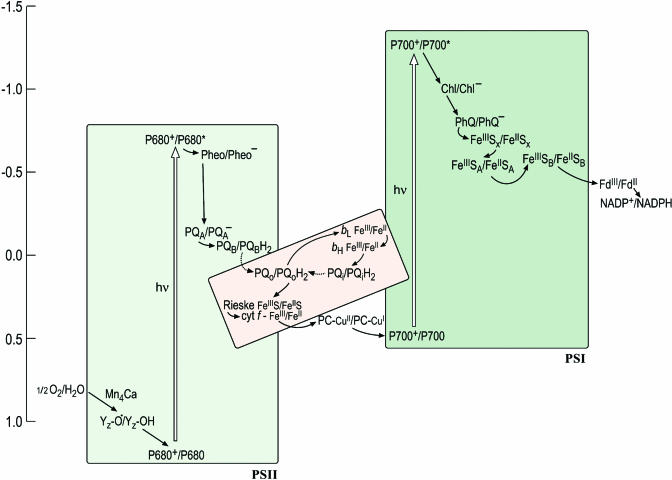
Figure 1.
An Updated Z-Scheme Describing Photosynthetic Electron Transfer.
Each carrier is shown in both its oxidized and reduced form to facilitate the readers' understanding of the sequence of steps, in which each carrier accepts an electron from a donor to become reduced and is reoxidized as it gives an electron to an acceptor. The midpoint potentials of the carriers in this version of the scheme have been updated to reflect the recent literature but are yet only approximate, owing in part to artistic aesthetics and in part to the intrinsic difficulty of estimating these numbers. In the cytochrome b6f complex (shown in pink to distinguish it as a heme-containing complex), the donor (reduced plastoquinol PQH2) provides two electrons: one is transferred through the Rieske FeS protein and cytochrome f to plastocyanin (or cytochrome c6) to photosystem I, while the other is transferred through the b-hemes to a bound quinone on the stromal side. The dashed arrow to and from PQH2 distinguishes diffusion of the redox carrier from the solid arrows that signify electron transfer. The conversion from the ground to the excited state (indicated with an open vertical arrow) occurs upon absorption of a photon. Some of the carriers appear to have obscure names (e.g., Z in PSII and A0 and A1 in PSI) and these have a historical origin in that the carriers had been identified as spectroscopic signals long before their chemical identities were known. In this scheme, A0 is indicated as Chl and A1 as PhQ to lead the reader away from obscurity. Redding and van der Est (2005) have suggested a specific nomenclature for the electron transfer cofactors in PSI.