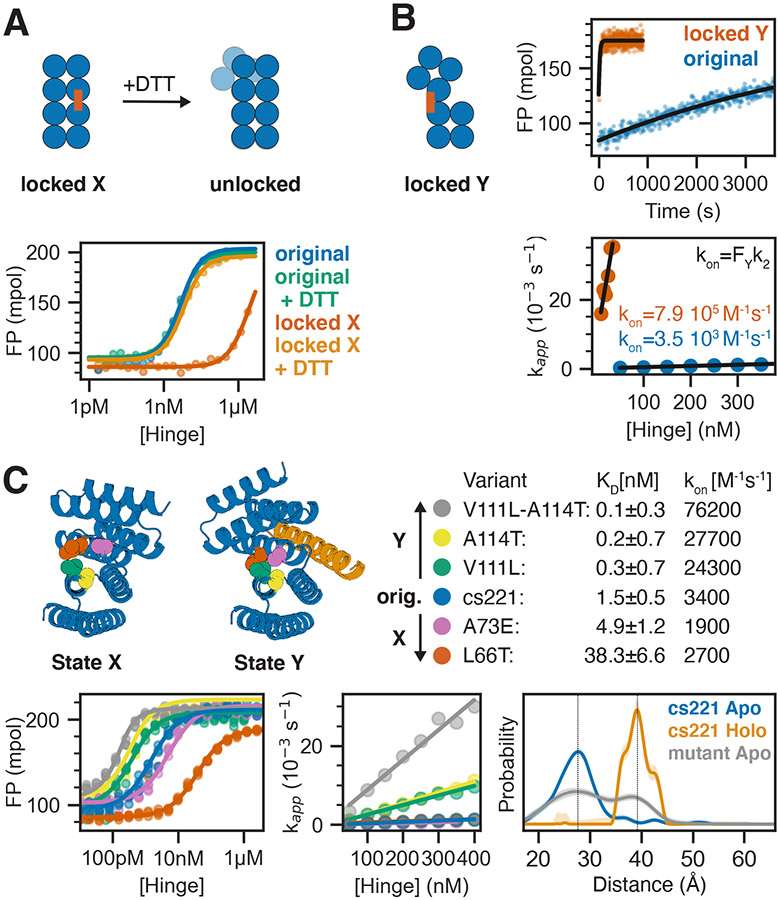
Figure 5: Controlling the conformational pre-equilibrium affects peptide binding.
A) Left: Schematic representation of a hinge containing two cysteine residues that can form a disulfide bond in state X but not in state Y, effectively locking the hinge in state X under oxidizing conditions. Upon addition of reducing agent DTT the disulfide bond is broken and the conformational equilibrium is restored. Right: FP-based titration of 1 nM TAMRA-labeled peptide and a hinge with state X disulfide (red, orange) or the parent hinge without cysteines (blue, green) under oxidizing (blue, red) or reducing (green, orange) conditions. B) From left to right: schematic representation of a hinge that is disulfide-locked in state Y; time course after mixing 2 nM TAMRA-labeled peptide and 50 nM locked hinge (red) or original hinge without cysteines (blue) fitted with a single-exponential equation (black line); apparent rate constants obtained from single-exponential kinetic fits plotted against absolute hinge concentrations (circles) and fitted with a linear equation (black line). C) Tuning the pre-equilibrium with point mutations. Top left: Cartoon representation of hinge cs221 highlighting positions of point mutations. Top right: Dissociation constants (KD) and observed binding rate constants (kon). Bottom left: FP-based titration of 0.1 nM (yellow, green, blue) or 1 nM (pink, red) TAMRA-labeled peptide cs221B and varying concentrations of hinge variants containing one or two point mutations. Bottom center: Apparent rate constants obtained from single-exponential kinetic fits plotted against absolute hinge concentrations (circles) and fitted with a linear equation (black line). Bottom right: DEER distance distribution for the double mutant cs221-V111L-A114T in absence of peptide (gray) in comparison to the original cs221 with (orange) and without (blue) peptide. Vertical lines serve as guide to the eye indicating state X and state Y distances.