Figure 1. Carrageenan and MIA pain models cause changes in ethological and evoked behavior accompanied by alterations in sensory neuron excitability over distinct timescales.
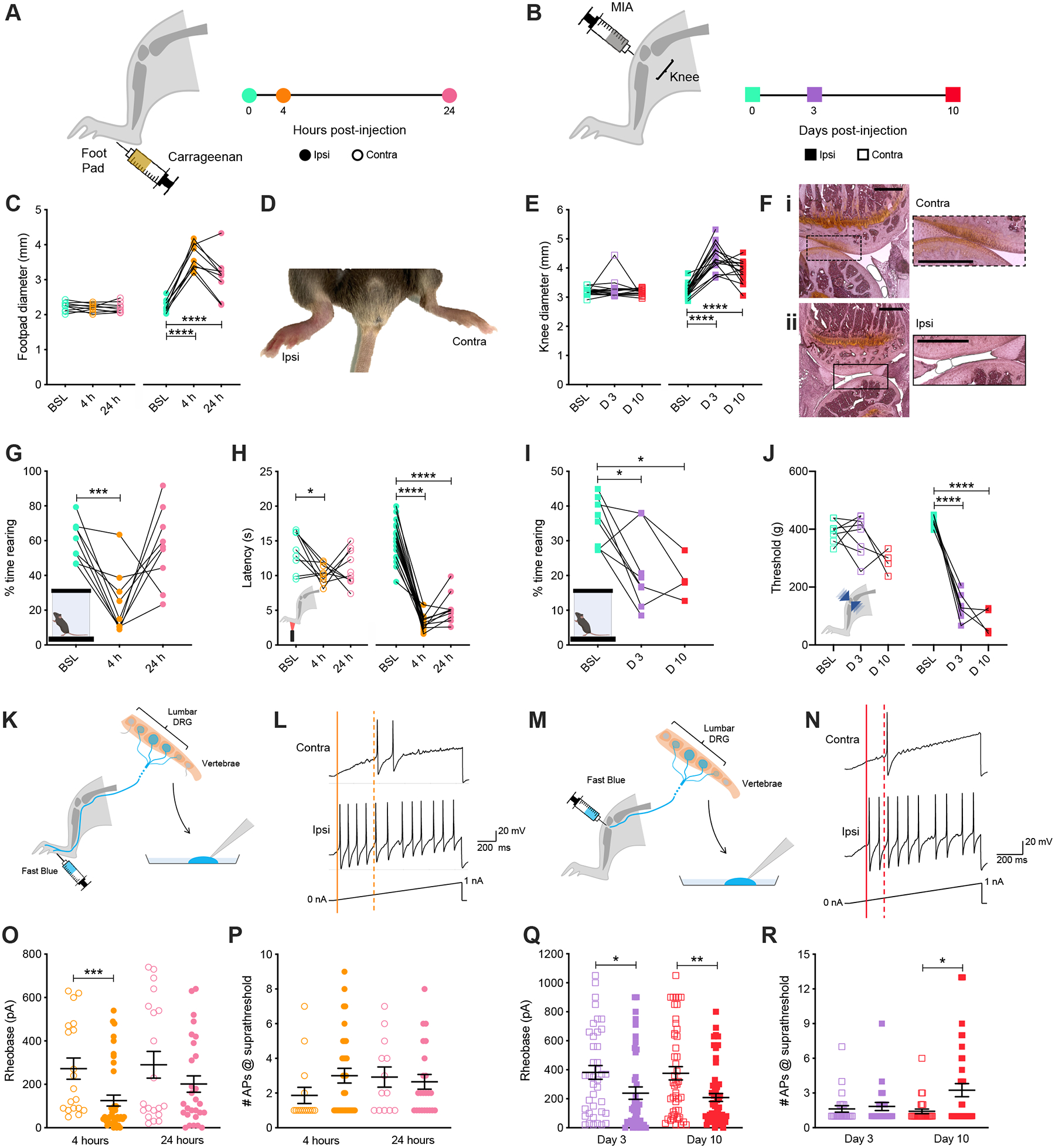
Timeline of experiments following (A) unilateral intraplantar injection of carrageenan or (B) intra-articular injection of MIA. (C) Inflammation of the injected (Ipsi) hind paw was observed 4-hours post-injection compared to the non-injected (Contra) paw. (D) Footpad swelling was quantified with digital calipers following injection of carrageenan. (E) Histological examination of knee joints 10 days after injection showed a healthy layer of cartilage for the contralateral joint (Ei), which had been lost in the ipsilateral joint (Eii). (F) Knee joint swelling was measured following injection of MIA. (G) The time mice spent rearing before, 4-hours, and 24-hours post-induction of inflammation with carrageenan was assessed using a dynamic weight bearing device. (H) Hargreaves measurement of carrageenan-induced heat hypersensitivity was assessed at baseline and following 4- and 24-hours. (I) Time spent rearing was assessed at baseline (BSL) and 3- (D 3) and 10-days (D 10) post-injection of MIA. (J) Sensitivity of both knee joints to mechanical stimulation was tested using a pressure application measurement device before and 3- and 10-days post-injection of MIA. (K) Schematic representation of retrograde labeling of hind paw innervating sensory neurons with Fast Blue followed by cell culture and whole cell patch clamp electrophysiology. (L) Representative current clamp recordings of Ipsi and Contra hind paw neurons of comparable capacitance, showing action potentials evoked by ramp injection of current (0–1 nA, 1 s), the thresholds for action potential discharge are annotated with dashed (Contra) or solid (Ipsi) lines. (M) Schematic representation of retrograde labeling of knee innervating sensory neurons with Fast Blue followed by cell culture and whole cell patch clamp electrophysiology. (N) Representative current clamp recordings of Ipsi and Contra knee neurons of comparable capacitance, showing action potentials evoked by ramp injection of current (0–1 nA, 1 s), the thresholds for action potential discharge are annotated with dashed (Contra) or solid (Ipsi) lines. (O) Step-wise current injections were used to determine the rheobase of Ipsi and Contra hind paw innervating sensory neurons 4- or 24-hours post-induction of inflammation with carrageenan. (P) Neurons with rheobase < 450 pA were stimulated with a suprathreshold (2 x rheobase) for 500 ms and the number of action potentials discharged counted. (O) Step-wise current injections were used to determine the rheobase of Ipsi and Contra knee innervating sensory neurons 3- or 10-days post-injection of MIA. (P) Neurons with rheobase < 450 pA were stimulated with a suprathreshold (2 x rheobase) for 500 ms and number of action potentials discharged counted. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001: (C, E, G, H, I, J) one-way ANOVA with Bonferroni post hoc; (O, Q, R) Mann-Whitney test between Ipsi and Contra for individual time points.
