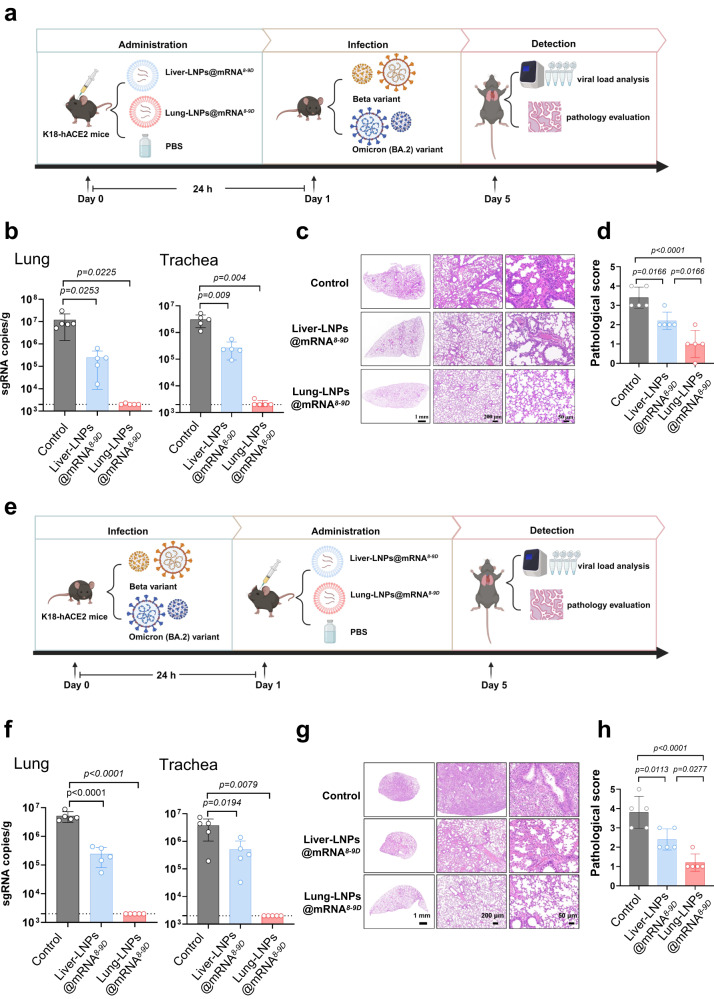
Fig. 5. 8-9D mRNA protective efficacy in SARS-CoV-2 animal model.
a–d 8-9D mRNA protective efficacy as prophylaxis, the K18-hACE2 mice (n = 5) were i.v. injected with 5 μg of Liver-LNPs@mRNA8-9D or Lung-LNPs@mRNA8-9D. Twenty-four hours post LNP injection, the mice were challenged with 2 × 104 TCID50 Beta variant. Four days post challenge, the mice were euthanized, and the lungs and tracheae were collected for viral load analysis by qRT–PCR, n = 5 biologically independent animals (b). The lungs were fixed for pathology evaluation (c), and pathological scores (d) were determined for significant comparison. PBS-injected mice were set as control, n = 5 biologically independent animals. e–h 8-9D mRNA protective efficacy as treatment. The mice were first anesthetized and intranasally inoculated with 2 × 104 TCID50 of authentic SARS-CoV-2 Beta variant, and after 24 h, the mice were intravenously administered one dose of 5 μg of Liver-LNPs@mRNA8-9D or Lung-LNPs@mRNA8-9D, or PBS alone as a control. The mice were euthanized 4 days post infection to harvest lung tissues and trachea tissues for viral load test (f) or histopathology evaluation (g, h). Data are presented as mean ± SD and representative of two independent experiments with similar results, n = 5 biologically independent animals. P values were determined by one-way ANOVA with Tukey’s multiple comparison post-hoc test. Source data are provided as a Source data file.