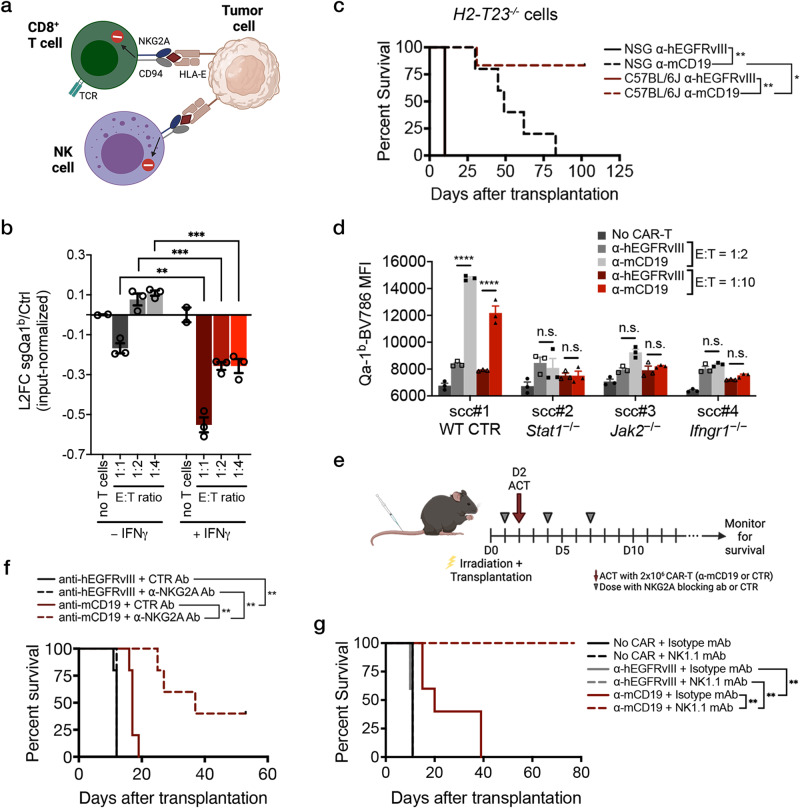
Fig. 4. Loss of Qa-1b, a component of the MHC-I pathway, or pharmacologic blockade of NKG2A, the only known receptor of Qa-1b, sensitizes B-ALL cells to CAR-T therapy in vivo.
a Schematic showing known effects of the HLA-E/NKG2A/CD94 axis in human NK and CD8+ T-cells. b In vitro competitive assays where CD19-expressing H2-T23−/− and CD19-expressing H2-T2+/+ pancreas cancer cells were mixed in a 50:50 ratio and exposed to the indicated concentration of anti-mCD19 CAR-T cells. The log2fold change (L2FC) of H2-T23−/− to control cells is shown. Cells were either pre-treated (right) with exogenous recombinant IFNγ or left untreated throughout the experiment (left). For the no T cells groups, n = 2 biologically independent samples; n = 5 for all other groups. c Immunocompetent (C57BL/6 J) mice transplanted with H2-T23−/− cells show increased survival compared to immunocompromised (NSG) mice transplanted with the same H2-T23−/− cells and treated with anti-mCD19 CAR-T cells (n = 5 mice per group). d Mean fluorescence intensity (MFI) of Qa-1b-Brilliant Violet 786 (BV786) after in vitro CAR-T treatment of single cell clones (scc) deficient in IFNγR/JAK/STAT pathway members at two different E:T ratios. Wildtype scc were also assayed, as shown. e Treatment schedule for the anti-NKG2A blocking antibody experiment. f A Kaplan-Meier curve showing overall survival in mice treated with a murine version of the anti-NKG2A antibody, monalizumab or a control antibody, concurrently with mCD19 or control hEGFRvIII CAR-T cells, as indicated. g Kaplan-Meier curves showing overall survival in mice treated concurrently with either anti-NK1.1 or isotype control antibodies along with either anti-mCD19, anti-hEGFRvIII, or no CAR-T cells. In this experiment, mice were pre-treated with anti-NK1.1 antibody one day prior to CAR-T therapy. The survival experiments and in vitro CAR-T treatment of H2-T23−/− cells were completed at least twice and were paired each time. Pharmacologic experiments were completed once. The significance of survival experiments was determined using log-rank tests. For all other experiments, significance was determined using unpaired two-sided student’s t-tests with Bonferroni correction for multiple comparisons. Data are mean ± s.e.m. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Exact P values for each comparison shown in (b–d) and (f–g) can be found in Supplementary Data 3.