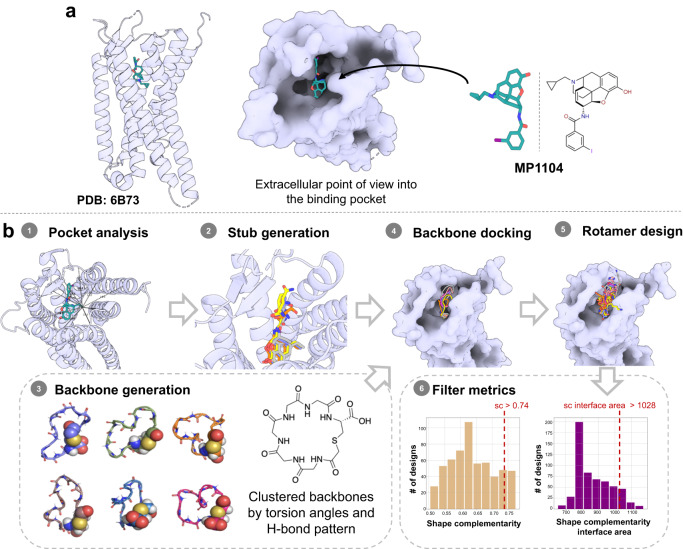
Fig. 1. Strategy for the computational design of thioether macrocyclized peptide–small molecule conjugates targeting KOR.
a 3D human KOR structure with small molecule agonist MP1104 was used as the starting template (PDB: 6B73). b Workflows for computational peptide–small molecule conjugate design: (1) Measurement of the pocket area (934 Å3) narrowed down the size of macrocycles to focus on 5- and 6-mer cyclic peptides. (2) Generation of small molecules with two additional amino acids, which were sampled and scored for optimal dimer sequence (select dipeptide modified small molecules: Gray: CVV-D-Phe-Thr; Yellow: CVV-D-Phe-Gln; Orange: CVV-D-Phe-Ser; CVV corresponds to N-cyclopropylmethyl-epoxy morphinan small molecule stub). (3) Generation of a comprehensive library of 5- and 6-mer thioether cyclized peptides clustered via torsion angle and hydrogen bond pattern. (4) Docked structure of thioether macrocyclized hexamers through coordinate-guided transformation of the backbone C-termini to the generated anchor N-termini. (5) Rotamer design to optimize the interface interactions of the backbones. (6) Design filtering based on shape complementarity and interface area as representative examples for interface metrics; dashed red line represents 90th percentile cut-off values.