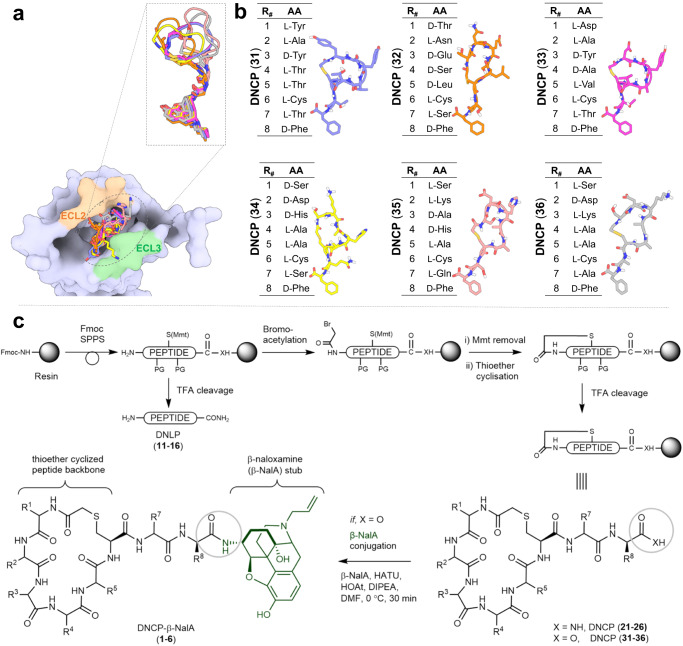
Fig. 2. Overview of the selected peptide macrocycle designs and the synthetic strategy to produce the peptide–small molecule conjugates and controls.
a Peptides have interactions with ECL2 and/or ECL3 of KOR with a subset overlay of the peptide backbone designs (aligned by the small molecule fragment anchor) showing shape diversity in the pocket. b Final selection of peptide sequences. Instead of focusing on multiple sequences for a single promising backbone, we sought to select designs across diverse shapes and sequences for experimental testing. c Synthetic scheme depicting solid phase synthesis of the de novo linear peptides (DNLP) (11–16) and the de novo cyclic peptides (DNCP) (21–26 and 31–36) and the solution phase conjugation reaction with β-naloxamine (β-NalA) to generate the DNCP-β-NalA (1–6) conjugates. R# indicates a side chain of the respective amino acid. PG denotes protecting groups. Rink amide resin was used for synthesis of DNLP (11–16) and DNCP (21–26), whereas Fmoc-D-Phe preloaded Wang resin was used for DNCP (31–36) synthesis. The amino acids indicated by R# in Fig. 2b correspond to the identical side chain represented by R# in Fig. 2c.