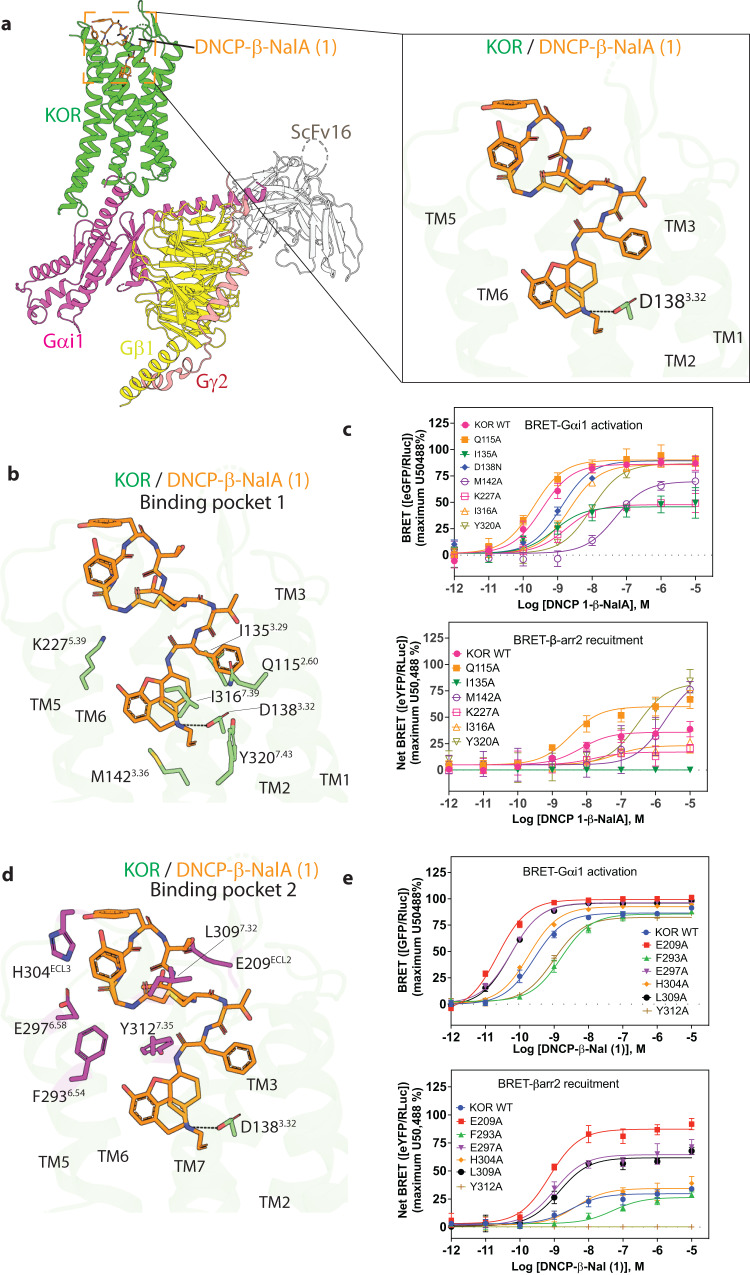
Fig. 5. Cryo-EM structure of the KOR-DNCP-β-NalA(1)-Gi1 complex.
a Overall architecture of the active state human KOR bound to DNCP-β-NalA(1) and G-protein heterotrimer (Gαi1, Gβ1, Gγ2). The KOR-G-protein complex was further stabilized by a single-chain antibody scFv16. The right panel shows the binding pose of DNCP-β-NalA(1) at KOR. The highly conserved anchoring residue D138, as part of the orthosteric binding pocket of KOR is shown. b The interactions between the bottom half of DNCP-β-NalA(1) and the orthosteric site of KOR. c Effects of orthosteric residues on DNCP-β-NalA(1)-mediated Gαi1 protein and β-arrestin-2 signaling (n = 3). For KOR D138N mutant, the reference ligand is salvinorin A because U50,488 is inactive at this mutant. d The interactions between the peptide macrocycle of DNCP-β-NalA(1) and the extracellular binding pocket 2 of KOR. e Effects of binding pocket 2 residues on DNCP-β-NalA(1)-mediated Gαi1 protein and β-arrestin-2 signaling (n = 3). All data are presented as mean values ± s.e.m. Source data are provided as a Source Data file.