Figure 2. Best Overall Responses Assessed by Blinded Independent Central Review.
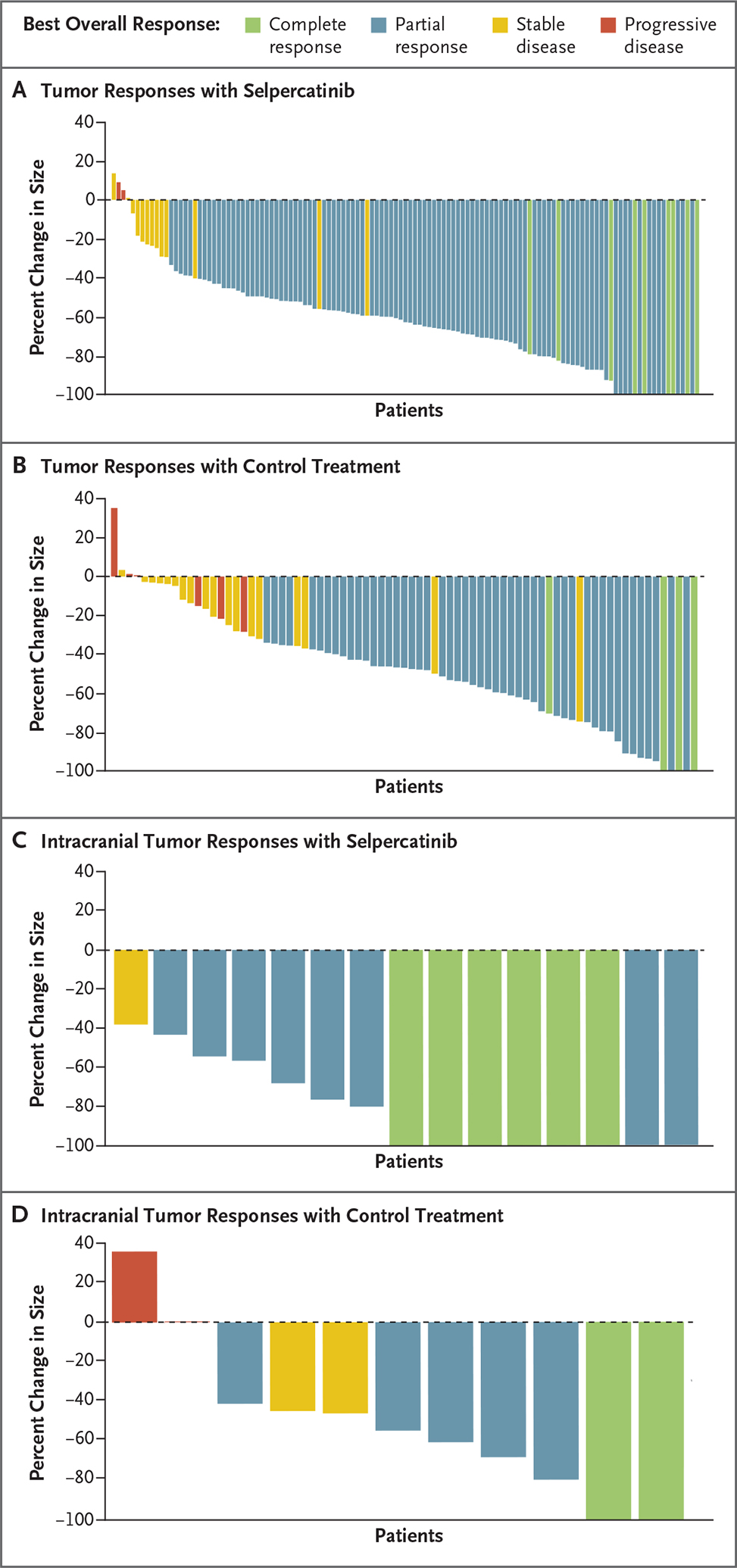
Panels A and B show waterfall plots of the maximum change from baseline in tumor size for patients with at least one evaluable postbaseline assessment according to blinded independent central review; data were available for 123 patients in the selpercatinib group and 77 patients in the control group in the intention-to-treat–pembrolizumab population. Panels C and D show waterfall plots of the maximum change from baseline in intracranial tumor size according to blinded independent central review for 15 patients in the selpercatinib group and 11 patients in the control group who had measurable brain metastases at baseline and at least one evaluable postbaseline assessment.
