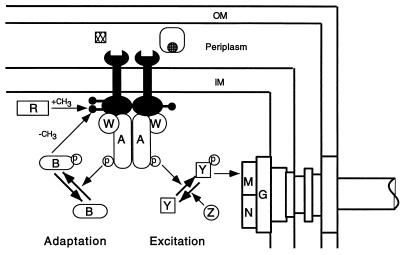
FIG. 2.
Circuit diagram of the chemotactic signaling pathway. The dimeric membrane-spanning chemoreceptors (paired black wrench-like objects) form a ternary complex with two CheA and two CheW polypeptides and stimulate the autokinase activity of CheA. CheA-P can transfer the phosphate to CheY. CheY-P interacts with FliM in the motor-switch complex to induce CW flagellar rotation. The decay of CheY-P is accelerated by CheZ. CheR is a constitutive methyltransferase that methylates certain glutamate residues in the cytoplasmic domains of the receptors. CheB is a methylesterase that is activated by phosphotransfer from CheA-P. CheB-P removes methyl groups from the receptors. In the excitation pathway, some attractant ligands (cross-hatched square) bind directly to the periplasmic domains of the receptors. Others (cross-hatched circle) first bind to substrate-binding proteins, which then interact with the periplasmic domains of the receptors. Attractant binding inhibits stimulation of CheA activity by the receptors. As a consequence, the CheY-P level rapidly falls, and CW flagellar rotation is suppressed. In the adaptation pathway, reduced CheA activity decreases the CheB-P level, although more slowly than the CheY-P level. As methylesterase activity declines, the receptors become more highly methylated. Increased methylation counteracts the attractant-dependent inhibition of CheA activity. As CheA activity rises, the intracellular CheY-P concentration returns to its prestimulus value, and the flagellar motor resumes its prestimulus CW-to-CCW switching ratio. Abbreviations: OM, outer membrane; IM, inner membrane; A, CheA; W, CheW; Y, CheY; Z, CheZ; R, CheR; B, CheB; G, FliG; M, FliM; N, FliN; p, phosphate; CH3, methyl group (shown as lollipop-like objects on the cytoplasmic domains of the receptors). Figure courtesy of Paul Gardina.