Figure 1. Patient Flow in the ORIENT-16 Trial.
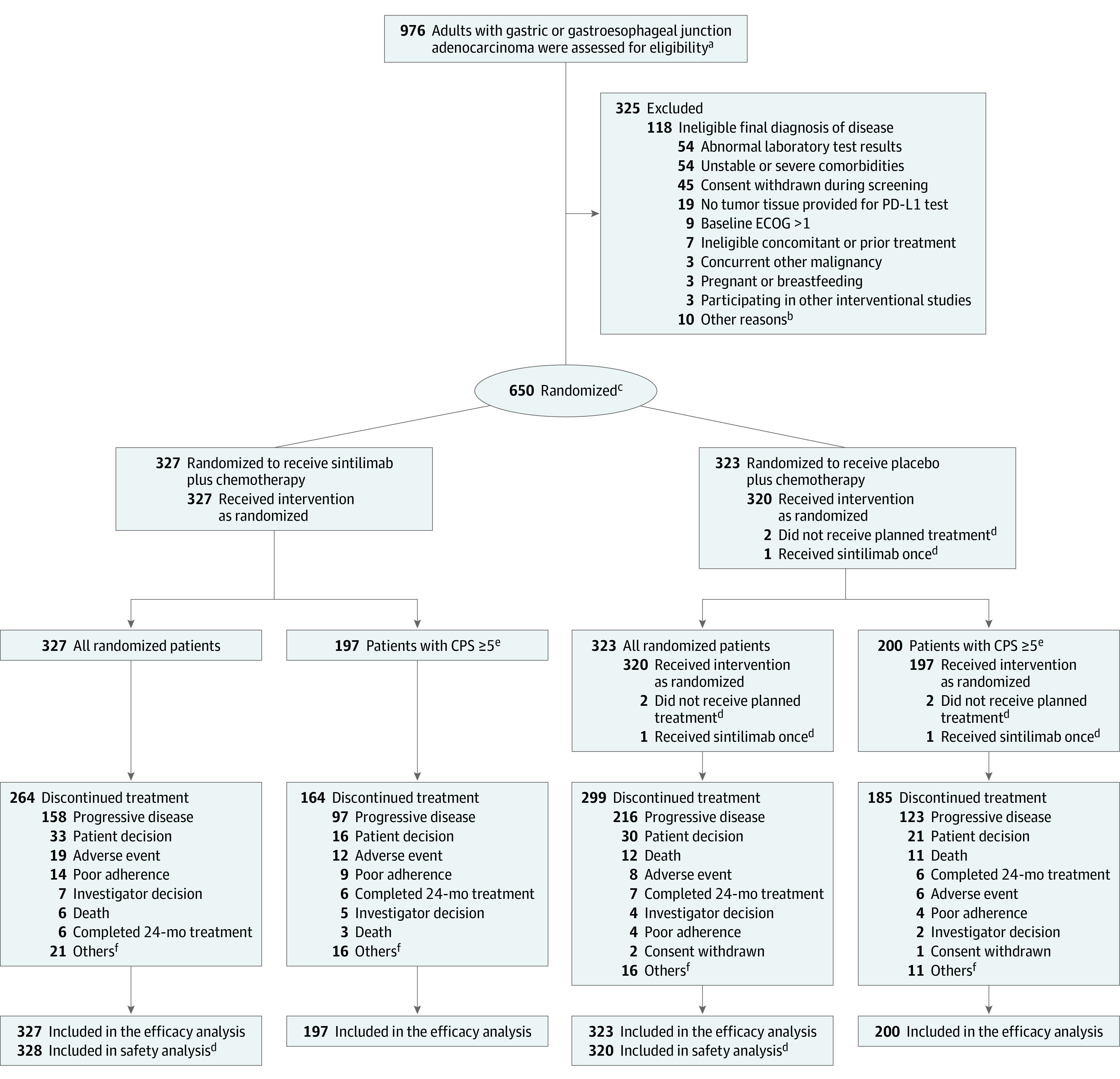
aThe total number of patients approached for participation in the trial was not recorded.
bOther reasons included a screening period exceeding 28 days or having an ineligible condition.
cPatients were randomized in a 1:1 ratio and stratified according to Eastern Cooperative Oncology Group performance status score (0 or 1), liver metastasis (yes or no), and programmed cell death ligand 1 (PD-L1) expression (combined positive score [CPS], <10 or ≥10). See Table 1 footnotes for more information. One patient who was assigned with 2 randomization numbers was counted as 1.
dFor the safety analysis, 1 patient who received 1 dose of sintilimab was analyzed in the sintilimab group, and 2 patients who did not receive planned treatment were excluded.
ePD-L1 expression was measured by CPS, which was defined as the number of PD-L1 staining cells (tumor cells, lymphocytes and macrophages) divided by the total number of viable tumor cells present in the sample multiplied by 100. The maximum score is defined as 100 when the calculation exceeds 100. A CPS of 5 or more was defined as PD-L1 positive.
fOther reasons included clinical progression, maximum benefit reached for patients who became eligible to undergo radical surgery after study treatment, and dose interruption up to the maximum duration of 12 weeks.
