Figure. Flow of Patients Through the Pediatric Adenotonsillectomy Trial for Snoring.
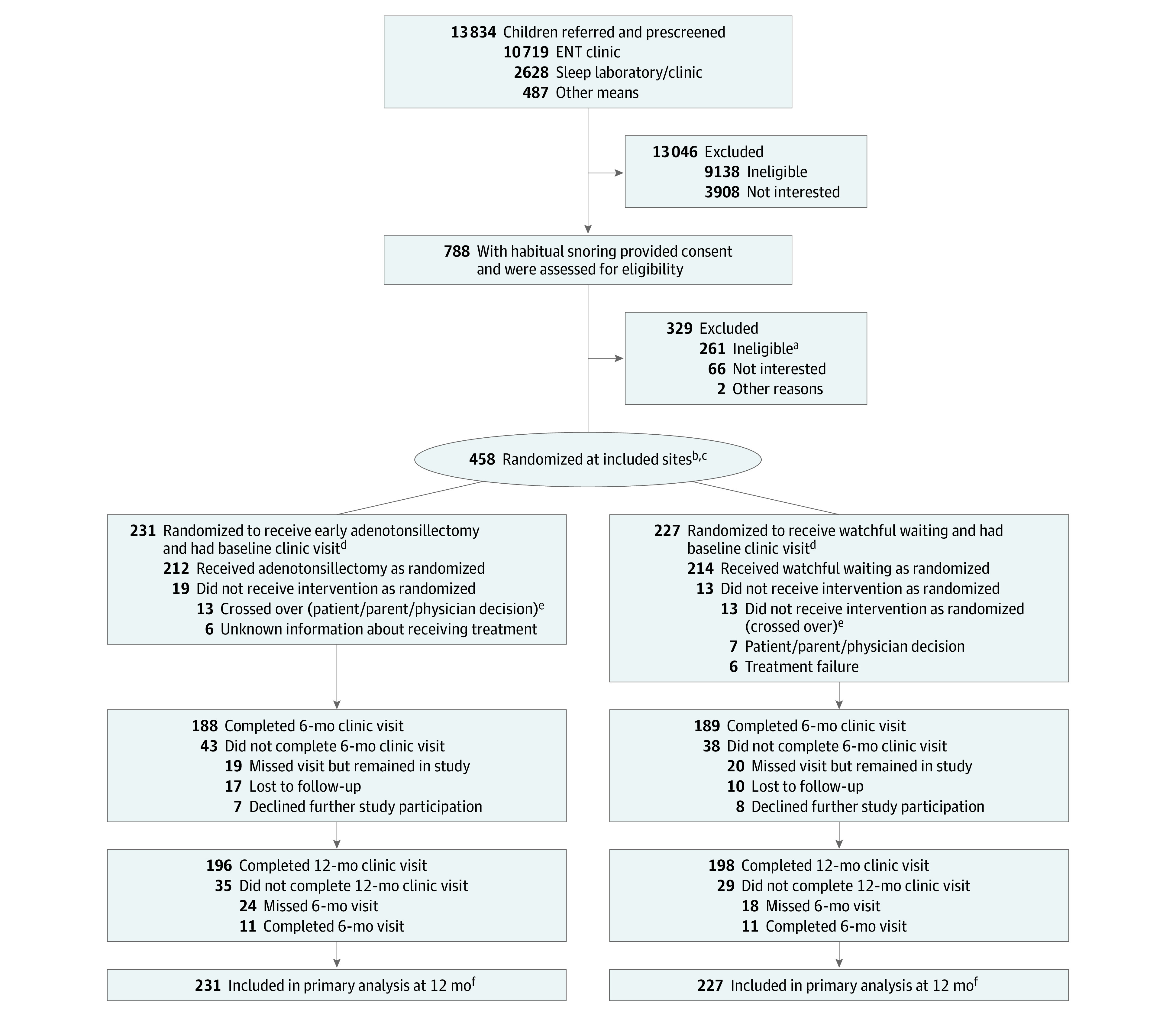
ENT indicates ear, nose, and throat.
aReasons for exclusion included apnea-hypopnea index level out of range; severe, chronic health problems; use of study-restricted medications; no report of habitual snoring; tonsillar size less than 2 on Brodsky scale; and lack of clinical equipoise.
bRandomization was stratified by age (≤5 vs >5 years), race (Black/African American vs other race or ethnicity), and body mass index (≤85 vs >85th percentile) within each study site.
cOne child randomized to watchful waiting was excluded due to site withdrawal but remained in the study and completed 12-month follow-up.
dVisit defined as completing either of the primary outcomes (BRIEF [Behavior Rating Inventory of Executive Function] or GNG [Go/No-go]).
eAnalyses reported are based on intention-to-treat; participants who crossed over were analyzed based on their randomized treatment assignments.
fThe primary analysis used a mixed-effects modeling approach that included observations from study participants at all included sites who had at least 1 measurement.
